PIAS1 modulates striatal transcription, DNA damage repair, and SUMOylation with relevance to Huntington's disease
- PMID: 33468657
- PMCID: PMC7848703
- DOI: 10.1073/pnas.2021836118
PIAS1 modulates striatal transcription, DNA damage repair, and SUMOylation with relevance to Huntington's disease
Erratum in
-
Correction for Morozko et al., PIAS1 modulates striatal transcription, DNA damage repair, and SUMOylation with relevance to Huntington's disease.Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2112001118. doi: 10.1073/pnas.2112001118. Proc Natl Acad Sci U S A. 2021. PMID: 34341126 Free PMC article. No abstract available.
Abstract
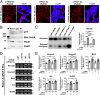
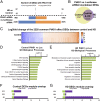
DNA damage repair genes are modifiers of disease onset in Huntington's disease (HD), but how this process intersects with associated disease pathways remains unclear. Here we evaluated the mechanistic contributions of protein inhibitor of activated STAT-1 (PIAS1) in HD mice and HD patient-derived induced pluripotent stem cells (iPSCs) and find a link between PIAS1 and DNA damage repair pathways. We show that PIAS1 is a component of the transcription-coupled repair complex, that includes the DNA damage end processing enzyme polynucleotide kinase-phosphatase (PNKP), and that PIAS1 is a SUMO E3 ligase for PNKP. Pias1 knockdown (KD) in HD mice had a normalizing effect on HD transcriptional dysregulation associated with synaptic function and disease-associated transcriptional coexpression modules enriched for DNA damage repair mechanisms as did reduction of PIAS1 in HD iPSC-derived neurons. KD also restored mutant HTT-perturbed enzymatic activity of PNKP and modulated genomic integrity of several transcriptionally normalized genes. The findings here now link SUMO modifying machinery to DNA damage repair responses and transcriptional modulation in neurodegenerative disease.
Keywords: DNA damage repair; Huntington's disease; PIAS; PNKP; SUMO.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- The Huntington’s Disease Collaborative Research Group , A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72, 971–983 (1993). - PubMed
-
- Langbehn D. R., Brinkman R. R., Falush D., Paulsen J. S., Hayden M. R.; International Huntington’s Disease Collaborative Group , A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 65, 267–277 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

