Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions
- PMID: 33468662
- PMCID: PMC7848718
- DOI: 10.1073/pnas.2023236118
Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions
Abstract
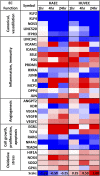
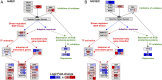
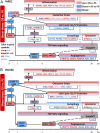
The two main blood flow patterns, namely, pulsatile shear (PS) prevalent in straight segments of arteries and oscillatory shear (OS) observed at branch points, are associated with atheroprotective (healthy) and atheroprone (unhealthy) vascular phenotypes, respectively. The effects of blood flow-induced shear stress on endothelial cells (ECs) and vascular health have generally been studied using human umbilical vein endothelial cells (HUVECs). While there are a few studies comparing the differential roles of PS and OS across different types of ECs at a single time point, there is a paucity of studies comparing the temporal responses between different EC types. In the current study, we measured OS and PS transcriptomic responses in human aortic endothelial cells (HAECs) over 24 h and compared these temporal responses of HAECs with our previous findings on HUVECs. The measurements were made at 1, 4, and 24 h in order to capture the responses at early, mid, and late time points after shearing. The results indicate that the responses of HAECs and HUVECs are qualitatively similar for endothelial function-relevant genes and several important pathways with a few exceptions, thus demonstrating that HUVECs can be used as a model to investigate the effects of shear on arterial ECs, with consideration of the differences. Our findings show that HAECs exhibit an earlier response or faster kinetics as compared to HUVECs. The comparative analysis of HAECs and HUVECs presented here offers insights into the mechanisms of common and disparate shear stress responses across these two major endothelial cell types.
Keywords: atherosclerosis; endothelial cells; systems biology; temporal analysis of flow response; transcriptomics.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Chistiakov D. A., Orekhov A. N., Bobryshev Y. V., Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol. (Oxf.) 219, 382–408 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

