Engineering a Reliable and Convenient SARS-CoV-2 Replicon System for Analysis of Viral RNA Synthesis and Screening of Antiviral Inhibitors
- PMID: 33468688
- PMCID: PMC7845634
- DOI: 10.1128/mBio.02754-20
Engineering a Reliable and Convenient SARS-CoV-2 Replicon System for Analysis of Viral RNA Synthesis and Screening of Antiviral Inhibitors
Abstract
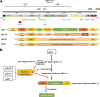
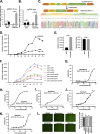
The etiologic agent of COVID-19 is highly contagious and has caused a severe global pandemic. Until now, there has been no simple and reliable system available in a lower-biosafety-grade laboratory for SARS-CoV-2 virologic research and inhibitor screening. In this study, we reported a replicon system which consists of four plasmids expressing the required segments of SARS-CoV-2. Our study revealed that the features for viral RNA synthesis and responses to antivirus drugs of the replicon are similar to those of wild-type viruses. Further analysis indicated that ORF6 provided potent in trans stimulation of the viral replication. Some viral variations, such as 5'UTR-C241T and ORF8-(T28144C) L84S mutation, also exhibit their different impact upon viral replication. Besides, the screening of clinically used drugs identified that several tyrosine kinase inhibitors and DNA-Top II inhibitors potently inhibit the replicon, as well as authentic SARS-CoV-2 viruses. Collectively, this replicon system provides a biosafety-worry-free platform for studying SARS-CoV-2 virology, monitoring the functional impact of viral mutations, and developing viral inhibitors.IMPORTANCE COVID-19 has caused a severe global pandemic. Until now, there has been no simple and reliable system available in a lower-biosafety-grade laboratory for SARS-CoV-2 virologic research and inhibitor screening. We reported a replicon system which consists of four ordinary plasmids expressing the required segments of SARS-CoV-2. Using the replicon system, we developed three application scenarios: (i) to identify the effects of viral proteins on virus replication, (ii) to identify the effects of mutations on viral replication during viral epidemics, and (iii) to perform high-throughput screening of antiviral drugs. Collectively, this replicon system would be useful for virologists to study SARS-CoV-2 virology, for epidemiologists to monitor virus mutations, and for industry to develop antiviral drugs.
Keywords: COVID-19; SARS-CoV-2; safety replicon.
Copyright © 2021 Luo et al.
Figures

Similar articles
-
Generation of SARS-CoV-2 reporter replicon for high-throughput antiviral screening and testing.Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2025866118. doi: 10.1073/pnas.2025866118. Proc Natl Acad Sci U S A. 2021. PMID: 33766889 Free PMC article.
-
Construction of a Noninfectious SARS-CoV-2 Replicon for Antiviral-Drug Testing and Gene Function Studies.J Virol. 2021 Aug 25;95(18):e0068721. doi: 10.1128/JVI.00687-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34191580 Free PMC article.
-
SARS-CoV-2 replicon for high-throughput antiviral screening.J Gen Virol. 2021 May;102(5):001583. doi: 10.1099/jgv.0.001583. J Gen Virol. 2021. PMID: 33956592 Free PMC article.
-
Reverse genetic systems of SARS-CoV-2 for antiviral research.Antiviral Res. 2023 Feb;210:105486. doi: 10.1016/j.antiviral.2022.105486. Epub 2022 Dec 22. Antiviral Res. 2023. PMID: 36657881 Free PMC article. Review.
-
Manipulation of genes could inhibit SARS-CoV-2 infection that causes COVID-19 pandemics.Exp Biol Med (Maywood). 2021 Jul;246(14):1643-1649. doi: 10.1177/15353702211008106. Epub 2021 Apr 25. Exp Biol Med (Maywood). 2021. PMID: 33899542 Free PMC article. Review.
Cited by
-
A Structurally Conserved RNA Element within SARS-CoV-2 ORF1a RNA and S mRNA Regulates Translation in Response to Viral S Protein-Induced Signaling in Human Lung Cells.J Virol. 2022 Jan 26;96(2):e0167821. doi: 10.1128/JVI.01678-21. Epub 2021 Nov 10. J Virol. 2022. PMID: 34757848 Free PMC article.
-
A Unique Robust Dual-Promoter-Driven and Dual-Reporter-Expressing SARS-CoV-2 Replicon: Construction and Characterization.Viruses. 2022 May 5;14(5):974. doi: 10.3390/v14050974. Viruses. 2022. PMID: 35632716 Free PMC article.
-
Comparative Analysis of SARS-CoV-2 Detection Kits.Acta Inform Med. 2022 Jun;30(2):110-114. doi: 10.5455/aim.2022.30.110-114. Acta Inform Med. 2022. PMID: 35774833 Free PMC article.
-
NSP6 inhibits the production of ACE2-containing exosomes to promote SARS-CoV-2 infectivity.mBio. 2024 Mar 13;15(3):e0335823. doi: 10.1128/mbio.03358-23. Epub 2024 Feb 2. mBio. 2024. PMID: 38303107 Free PMC article.
-
Application of emetine in SARS-CoV-2 treatment: regulation of p38 MAPK signaling pathway for preventing emetine-induced cardiac complications.Cell Cycle. 2022 Nov;21(22):2379-2386. doi: 10.1080/15384101.2022.2100575. Epub 2022 Jul 19. Cell Cycle. 2022. PMID: 35852390 Free PMC article.
References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi:10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi:10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Harcourt BH, Jukneliene D, Kanjanahaluethai A, Bechill J, Severson KM, Smith CM, Rota PA, Baker SC. 2004. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J Virol 78:13600–13612. doi:10.1128/JVI.78.24.13600-13612.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

