Trehalose Recycling Promotes Energy-Efficient Biosynthesis of the Mycobacterial Cell Envelope
- PMID: 33468692
- PMCID: PMC7845637
- DOI: 10.1128/mBio.02801-20
Trehalose Recycling Promotes Energy-Efficient Biosynthesis of the Mycobacterial Cell Envelope
Abstract
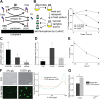
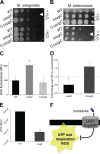
The mycomembrane layer of the mycobacterial cell envelope is a barrier to environmental, immune, and antibiotic insults. There is considerable evidence of mycomembrane plasticity during infection and in response to host-mimicking stresses. Since mycobacteria are resource and energy limited under these conditions, it is likely that remodeling has distinct requirements from those of the well-characterized biosynthetic program that operates during unrestricted growth. Unexpectedly, we found that mycomembrane remodeling in nutrient-starved, nonreplicating mycobacteria includes synthesis in addition to turnover. Mycomembrane synthesis under these conditions occurs along the cell periphery, in contrast to the polar assembly of actively growing cells, and both liberates and relies on the nonmammalian disaccharide trehalose. In the absence of trehalose recycling, de novo trehalose synthesis fuels mycomembrane remodeling. However, mycobacteria experience ATP depletion, enhanced respiration, and redox stress, hallmarks of futile cycling and the collateral dysfunction elicited by some bactericidal antibiotics. Inefficient energy metabolism compromises the survival of trehalose recycling mutants in macrophages. Our data suggest that trehalose recycling alleviates the energetic burden of mycomembrane remodeling under stress. Cell envelope recycling pathways are emerging targets for sensitizing resource-limited bacterial pathogens to host and antibiotic pressure.IMPORTANCE The glucose-based disaccharide trehalose is a stress protectant and carbon source in many nonmammalian cells. Mycobacteria are relatively unique in that they use trehalose for an additional, extracytoplasmic purpose: to build their outer "myco" membrane. In these organisms, trehalose connects mycomembrane biosynthesis and turnover to central carbon metabolism. Key to this connection is the retrograde transporter LpqY-SugABC. Unexpectedly, we found that nongrowing mycobacteria synthesize mycomembrane under carbon limitation but do not require LpqY-SugABC. In the absence of trehalose recycling, compensatory anabolism allows mycomembrane biosynthesis to continue. However, this workaround comes at a cost, namely, ATP consumption, increased respiration, and oxidative stress. Strikingly, these phenotypes resemble those elicited by futile cycles and some bactericidal antibiotics. We demonstrate that inefficient energy metabolism attenuates trehalose recycling mutant Mycobacterium tuberculosis in macrophages. Energy-expensive macromolecule biosynthesis triggered in the absence of recycling may be a new paradigm for boosting host activity against bacterial pathogens.
Keywords: Mycobacterium; mycomembrane; oxidative stress; starvation; trehalose.
Copyright © 2021 Pohane et al.
Figures




References
-
- Yang Y, Kulka K, Montelaro RC, Reinhart TA, Sissons J, Aderem A, Ojha AK. 2014. A hydrolase of trehalose dimycolate induces nutrient influx and stress sensitivity to balance intracellular growth of Mycobacterium tuberculosis. Cell Host Microbe 15:153–163. doi: 10.1016/j.chom.2014.01.008. - DOI - PMC - PubMed
-
- Galagan JE, Minch K, Peterson M, Lyubetskaya A, Azizi E, Sweet L, Gomes A, Rustad T, Dolganov G, Glotova I, Abeel T, Mahwinney C, Kennedy AD, Allard R, Brabant W, Krueger A, Jaini S, Honda B, Yu WH, Hickey MJ, Zucker J, Garay C, Weiner B, Sisk P, Stolte C, Winkler JK, Van de Peer Y, Iazzetti P, Camacho D, Dreyfuss J, Liu Y, Dorhoi A, Mollenkopf HJ, Drogaris P, Lamontagne J, Zhou Y, Piquenot J, Park ST, Raman S, Kaufmann SH, Mohney RP, Chelsky D, Moody DB, Sherman DR, Schoolnik GK. 2013. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499:178–183. doi: 10.1038/nature12337. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
