5-Aminosalicylic Acid Ameliorates Colitis and Checks Dysbiotic Escherichia coli Expansion by Activating PPAR-γ Signaling in the Intestinal Epithelium
- PMID: 33468700
- PMCID: PMC7845635
- DOI: 10.1128/mBio.03227-20
5-Aminosalicylic Acid Ameliorates Colitis and Checks Dysbiotic Escherichia coli Expansion by Activating PPAR-γ Signaling in the Intestinal Epithelium
Abstract
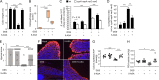
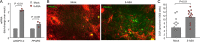
5-Aminosalicylic acid (5-ASA), a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist, is a widely used first-line medication for the treatment of ulcerative colitis, but its anti-inflammatory mechanism is not fully resolved. Here, we show that 5-ASA ameliorates colitis in dextran sulfate sodium (DSS)-treated mice by activating PPAR-γ signaling in the intestinal epithelium. DSS-induced colitis was associated with a loss of epithelial hypoxia and a respiration-dependent luminal expansion of Escherichia coli, which could be ameliorated by treatment with 5-ASA. However, 5-ASA was no longer able to reduce inflammation, restore epithelial hypoxia, or blunt an expansion of E. coli in DSS-treated mice that lacked Pparg expression specifically in the intestinal epithelium. These data suggest that the anti-inflammatory activity of 5-ASA requires activation of epithelial PPAR-γ signaling, thus pointing to the intestinal epithelium as a potential target for therapeutic intervention in ulcerative colitis.IMPORTANCE An expansion of Enterobacterales in the fecal microbiota is a microbial signature of dysbiosis that is linked to many noncommunicable diseases, including ulcerative colitis. Here, we used Escherichia coli, a representative of the Enterobacterales, to show that its dysbiotic expansion during colitis can be remediated by modulating host epithelial metabolism. Dextran sulfate sodium (DSS)-induced colitis reduced mitochondrial activity in the colonic epithelium, thereby increasing the amount of oxygen available to fuel an E. coli expansion through aerobic respiration. Activation of epithelial peroxisome proliferator-activated receptor gamma (PPAR-γ) signaling with 5-aminosalicylic acid (5-ASA) was sufficient to restore mitochondrial activity and blunt a dysbiotic E. coli expansion. These data identify the host's epithelial metabolism as a potential treatment target to remediate microbial signatures of dysbiosis, such as a dysbiotic E. coli expansion in the fecal microbiota.
Keywords: Escherichia coli; dysbiosis; gut inflammation; inflammatory bowel disease; microbial communities.
Copyright © 2021 Cevallos et al.
Figures


References
-
- Adeolu M, Alnajar S, Naushad S, Gupta RS. 2016. Genome-based phylogeny and taxonomy of the 'Enterobacteriales': proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int J Syst Evol Microbiol 66:5575–5599. doi: 10.1099/ijsem.0.001485. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007673/DK/NIDDK NIH HHS/United States
- R56 AI112949/AI/NIAID NIH HHS/United States
- R01 AI044170/AI/NIAID NIH HHS/United States
- R01 AI149632/AI/NIAID NIH HHS/United States
- R21 AI146432/AI/NIAID NIH HHS/United States
- R01 AI096528/AI/NIAID NIH HHS/United States
- R01 AI112949/AI/NIAID NIH HHS/United States
- R21 AI153069/AI/NIAID NIH HHS/United States
- R01 AI109799/AI/NIAID NIH HHS/United States
- R21 AI143253/AI/NIAID NIH HHS/United States
- TL1 TR000133/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- P50 CA236733/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
