Evidence-Based Guidelines for the Treatment of Helicobacter pylori Infection in Korea 2020
- PMID: 33468712
- PMCID: PMC7960974
- DOI: 10.5009/gnl20288
Evidence-Based Guidelines for the Treatment of Helicobacter pylori Infection in Korea 2020
Abstract
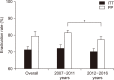
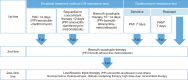
Helicobacter pylori infection is one of the most common infectious diseases worldwide. Although the prevalence of H. pylori is gradually decreasing, approximately half of the world's population still becomes infected with this disease. H. pylori is responsible for substantial gastrointestinal morbidity worldwide, with a high disease burden. It is the most common cause of gastric and duodenal ulcers and gastric cancer. Since the revision of the H. pylori clinical practice guidelines in 2013 in Korea, the eradication rate of H. pylori has gradually decreased with the use of a clarithromycin-based triple therapy for 7 days. According to a nationwide randomized controlled study conducted by the Korean College of Helicobacter and Upper Gastrointestinal Research released in 2018, the intention-to-treat eradication rate was only 63.9%, which was mostly due to increased antimicrobial resistance, especially from clarithromycin. The clinical practice guidelines for the treatment of H. pylori were updated according to evidence-based medicine from a meta-analysis conducted on a target group receiving the latest level of eradication therapy. The draft recommendations developed based on the meta-analysis were finalized after an expert consensus on three recommendations regarding the indication for treatment and eight recommendations for the treatment itself. These guidelines were designed to provide clinical evidence for the treatment (including primary care treatment) of H. pylori infection to patients, nurses, medical school students, policymakers, and clinicians. These may differ from current medical insurance standards and will be revised if more evidence emerges in the future.
Keywords: Helicobacter pylori; Guidelines; Meta-analysis; Microbial sensitivity tests; Treatment.
Conflict of interest statement
Y.C.L., a member of the Editor-in-Chief of
Figures
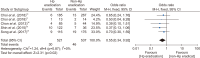






References
-
- Hunt RH, Xiao SD, Megraud F, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011;20:299–304. - PubMed
-
- Statistics Korea, author. Vital Statistics of Korea PeriodAnnual 1970-2018. Statistics Korea; Daejeon: c2019. [cited 2020 Nov 25]. Available from: http://kostat.go.kr/wnsearch/search.jsp .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

