Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma
- PMID: 33469002
- PMCID: PMC7815781
- DOI: 10.1038/s41467-020-20599-x
Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma
Abstract
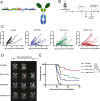
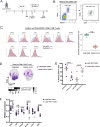
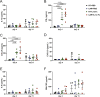
Glioblastoma multiforme (GBM) is the most common and aggressive form of primary brain cancer, for which effective therapies are urgently needed. Chimeric antigen receptor (CAR)-based immunotherapy represents a promising therapeutic approach, but it is often impeded by highly immunosuppressive tumor microenvironments (TME). Here, in an immunocompetent, orthotopic GBM mouse model, we show that CAR-T cells targeting tumor-specific epidermal growth factor receptor variant III (EGFRvIII) alone fail to control fully established tumors but, when combined with a single, locally delivered dose of IL-12, achieve durable anti-tumor responses. IL-12 not only boosts cytotoxicity of CAR-T cells, but also reshapes the TME, driving increased infiltration of proinflammatory CD4+ T cells, decreased numbers of regulatory T cells (Treg), and activation of the myeloid compartment. Importantly, the immunotherapy-enabling benefits of IL-12 are achieved with minimal systemic effects. Our findings thus show that local delivery of IL-12 may be an effective adjuvant for CAR-T cell therapy for GBM.
Conflict of interest statement
B.B. holds shares to Gaeta Therapeutics and a patent on the use of IL-12 tumor targeting in combination with check-point inhibition. The patent is named “COMBINATION MEDICAMENT COMPRISING IL-12 AND AN AGENT FOR BLOCKADE OF T-CELL INHIBITORY MOLECULES FOR TUMOUR THERAPY” with publication number: 20150017121. M.A.P. owns stock, receives salary contribution and research funding from Autolus Ltd. M.A.P. is entitled to a share of royalties earned from patents filed by UCL on his behalf. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

