Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development
- PMID: 33469018
- PMCID: PMC7815709
- DOI: 10.1038/s41467-020-20666-3
Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development
Abstract
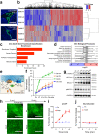
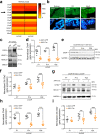
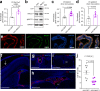
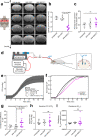
Cerebrospinal fluid (CSF) provides vital support for the brain. Abnormal CSF accumulation, such as hydrocephalus, can negatively affect perinatal neurodevelopment. The mechanisms regulating CSF clearance during the postnatal critical period are unclear. Here, we show that CSF K+, accompanied by water, is cleared through the choroid plexus (ChP) during mouse early postnatal development. We report that, at this developmental stage, the ChP showed increased ATP production and increased expression of ATP-dependent K+ transporters, particularly the Na+, K+, Cl-, and water cotransporter NKCC1. Overexpression of NKCC1 in the ChP resulted in increased CSF K+ clearance, increased cerebral compliance, and reduced circulating CSF in the brain without changes in intracranial pressure in mice. Moreover, ChP-specific NKCC1 overexpression in an obstructive hydrocephalus mouse model resulted in reduced ventriculomegaly. Collectively, our results implicate NKCC1 in regulating CSF K+ clearance through the ChP in the critical period during postnatal neurodevelopment in mice.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Age-appropriate potassium clearance from perinatal cerebrospinal fluid depends on choroid plexus NKCC1.Fluids Barriers CNS. 2023 Jun 16;20(1):45. doi: 10.1186/s12987-023-00438-z. Fluids Barriers CNS. 2023. PMID: 37328833 Free PMC article.
-
The Na+,K+,2Cl- Cotransporter, Not Aquaporin 1, Sustains Cerebrospinal Fluid Secretion While Controlling Brain K+ Homeostasis.Adv Sci (Weinh). 2025 Feb;12(6):e2409120. doi: 10.1002/advs.202409120. Epub 2024 Dec 18. Adv Sci (Weinh). 2025. PMID: 39692709 Free PMC article.
-
Elevated CSF inflammatory markers in patients with idiopathic normal pressure hydrocephalus do not promote NKCC1 hyperactivity in rat choroid plexus.Fluids Barriers CNS. 2021 Dec 4;18(1):54. doi: 10.1186/s12987-021-00289-6. Fluids Barriers CNS. 2021. PMID: 34863228 Free PMC article.
-
Cerebrospinal fluid hypersecretion in pediatric hydrocephalus.Neurosurg Focus. 2016 Nov;41(5):E10. doi: 10.3171/2016.8.FOCUS16278. Neurosurg Focus. 2016. PMID: 27798982 Review.
-
Idiopathic cerebrospinal fluid overproduction: case-based review of the pathophysiological mechanism implied in the cerebrospinal fluid production.Croat Med J. 2014 Aug 28;55(4):377-87. doi: 10.3325/cmj.2014.55.377. Croat Med J. 2014. PMID: 25165051 Free PMC article. Review.
Cited by
-
Choroid plexus-CSF-targeted antioxidant therapy protects the brain from toxicity of cancer chemotherapy.Neuron. 2022 Oct 19;110(20):3288-3301.e8. doi: 10.1016/j.neuron.2022.08.009. Epub 2022 Sep 6. Neuron. 2022. PMID: 36070751 Free PMC article.
-
Measurements of cerebrospinal fluid production: a review of the limitations and advantages of current methodologies.Fluids Barriers CNS. 2022 Dec 15;19(1):101. doi: 10.1186/s12987-022-00382-4. Fluids Barriers CNS. 2022. PMID: 36522656 Free PMC article. Review.
-
Activation of the RARα Attenuated CSF Hypersecretion to Inhibit Hydrocephalus Development via Regulating the MAFB/MSR1 Pathway.Int J Mol Sci. 2023 Jan 30;24(3):2586. doi: 10.3390/ijms24032586. Int J Mol Sci. 2023. PMID: 36768908 Free PMC article.
-
The choroid plexus: a missing link in our understanding of brain development and function.Physiol Rev. 2023 Jan 1;103(1):919-956. doi: 10.1152/physrev.00060.2021. Epub 2022 Sep 29. Physiol Rev. 2023. PMID: 36173801 Free PMC article. Review.
-
Ventricular-subventricular zone stem cell niche adaptations in a mouse model of post-infectious hydrocephalus.Front Neurosci. 2024 Jul 31;18:1429829. doi: 10.3389/fnins.2024.1429829. eCollection 2024. Front Neurosci. 2024. PMID: 39145299 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

