Host development overwhelms environmental dispersal in governing the ecological succession of zebrafish gut microbiota
- PMID: 33469034
- PMCID: PMC7815754
- DOI: 10.1038/s41522-020-00176-2
Host development overwhelms environmental dispersal in governing the ecological succession of zebrafish gut microbiota
Abstract
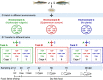
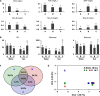
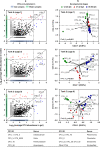
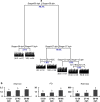
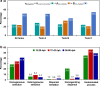
Clarifying mechanisms underlying the ecological succession of gut microbiota is a central theme of gut ecology. Under experimental manipulations of zebrafish hatching and rearing environments, we test our core hypothesis that the host development will overwhelm environmental dispersal in governing fish gut microbial community succession due to host genetics, immunology, and gut nutrient niches. We find that zebrafish developmental stage substantially explains the gut microbial community succession, whereas the environmental effects do not significantly affect the gut microbiota succession from larvae to adult fish. The gut microbiotas of zebrafish are clearly separated according to fish developmental stages, and the degree of homogeneous selection governing gut microbiota succession is increasing with host development. This study advances our mechanistic understanding of the gut microbiota assembly and succession by integrating the host and environmental effects, which also provides new insights into the gut ecology of other aquatic animals.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

