A real-world longitudinal study of anemia management in non-dialysis-dependent chronic kidney disease patients: a multinational analysis of CKDopps
- PMID: 33469061
- PMCID: PMC7815803
- DOI: 10.1038/s41598-020-79254-6
A real-world longitudinal study of anemia management in non-dialysis-dependent chronic kidney disease patients: a multinational analysis of CKDopps
Abstract
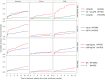
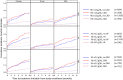
Previously lacking in the literature, we describe longitudinal patterns of anemia prescriptions for non-dialysis-dependent chronic kidney disease (NDD-CKD) patients under nephrologist care. We analyzed data from 2818 Stage 3-5 NDD-CKD patients from Brazil, Germany, and the US, naïve to anemia medications (oral iron, intravenous [IV] iron, or erythropoiesis stimulating agent [ESA]) at enrollment in the CKDopps. We report the cumulative incidence function (CIF) of medication initiation stratified by baseline characteristics. Even in patients with hemoglobin (Hb) < 10 g/dL, the CIF at 12 months for any anemia medication was 40%, and 28% for ESAs. Patients with TSAT < 20% had a CIF of 26% and 6% for oral and IV iron, respectively. Heart failure was associated with earlier initiation of anemia medications. IV iron was prescribed to < 10% of patients with iron deficiency. Only 40% of patients with Hb < 10 g/dL received any anemia medication within a year. Discontinuation of anemia treatment was very common. Anemia treatment is initiated in a limited number of NDD-CKD patients, even in those with guideline-based indications to treat. Hemoglobin trajectory and a history of heart failure appear to guide treatment start. These results support the concept that anemia is sub-optimally managed among NDD-CKD patients in the real-world setting.
Conflict of interest statement
James Sloand is employed by and owns stock in AstraZeneca; he owns stock options and shares in Baxter Healthcare Corporation. Michelle Wong was previously a consultant for Arbor Research Collaborative for Health; she has received research funding from Michael Smith Foundation for Health Research. Glen James is an employee of AstraZeneca who receives salary and owns stock. Ziad Massy reports grants and other from Amgen, grants and other from Sanofi-Genzyme, grants from French Government , grants from MSD, grants from GSK, grants from Lilly, grants from FMC, grants and other from Baxter, grants from Outsuka, other from Daichi, other from Astellas , outside the submitted work. Helmut Reichel reports honoraria from Vifor, Amgen, Hexal. Bryce Foote is an employee of Keryx Biopharmaceuticals. Katarina Hedman is employed by AstraZeneca and owns shares of AstraZeneca. Sandra Waechter is an employee of Vifor Pharma. Bruce M. Robinson, Jarcy Zee, Charlotte Tu, Murilo Guedes, Marcelo Barreto Lopes, Ronald L Pisoni, and Roberto Pecoits-Filho are employees of Arbor Research Collaborative for Health, which administers the DOPPS. Antonio A. Lopes does not have any conflicts of interest to declare.
Figures



References
-
- United States Renal Data System. 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, https://www.usrds.org/adr.aspx (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

