The antimicrobial potential of cannabidiol
- PMID: 33469147
- PMCID: PMC7815910
- DOI: 10.1038/s42003-020-01530-y
The antimicrobial potential of cannabidiol
Abstract
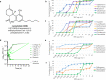
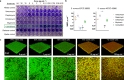
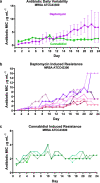
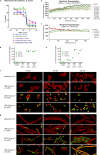
Antimicrobial resistance threatens the viability of modern medicine, which is largely dependent on the successful prevention and treatment of bacterial infections. Unfortunately, there are few new therapeutics in the clinical pipeline, particularly for Gram-negative bacteria. We now present a detailed evaluation of the antimicrobial activity of cannabidiol, the main non-psychoactive component of cannabis. We confirm previous reports of Gram-positive activity and expand the breadth of pathogens tested, including highly resistant Staphylococcus aureus, Streptococcus pneumoniae, and Clostridioides difficile. Our results demonstrate that cannabidiol has excellent activity against biofilms, little propensity to induce resistance, and topical in vivo efficacy. Multiple mode-of-action studies point to membrane disruption as cannabidiol's primary mechanism. More importantly, we now report for the first time that cannabidiol can selectively kill a subset of Gram-negative bacteria that includes the 'urgent threat' pathogen Neisseria gonorrhoeae. Structure-activity relationship studies demonstrate the potential to advance cannabidiol analogs as a much-needed new class of antibiotics.
Conflict of interest statement
The authors declare the following competing interests: M.C. and M.T. are employees of Botanix Inc, who co-funded most of this research. M.A.T.B. consults for Botanix Inc., and is an inventor on several antibiotic patents (unrelated to this work) which are undergoing commercialization. D.Q. and M.D.S. are employees and J.P. founded Linneas (conducted the bacterial cytological profiling). J.P. has an equity interest in Linnaeus Bioscience Incorporated and receives consulting income from the company. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. N.B. is founder of BDG Synthesis (conducted the analog synthesis), and D.W.C. is an employee and M.L.P. founded Extherid Biosciences (conducted the ex-vivo pig skin studies), which is now part of Perfectus Biomed Group. The remaining authors declare no competing interests.
Figures

References
-
- U.S. Department of Health and Human Services, CDC. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA. (2019).
-
- Roope LSJ, et al. The challenge of antimicrobial resistance: What economics can contribute. Nat. Rev. Drug Discov. 2019;364:eaau4679. - PubMed
-
- Årdal, C. et al DRIVE-AB REPORT. Revitalizing theantibiotic pipeline. Stimulating Innovation While Driving Sustainable Use and Global Access. (2018).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

