Overcoming the challenges associated with CD3+ T-cell redirection in cancer
- PMID: 33469153
- PMCID: PMC7960983
- DOI: 10.1038/s41416-020-01225-5
Overcoming the challenges associated with CD3+ T-cell redirection in cancer
Abstract
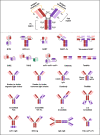
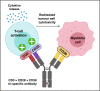
The development of bispecific antibodies that redirect the cytotoxic activity of CD3+ T cells to tumours is a promising immunotherapeutic strategy for the treatment of haematological malignancies and solid cancers. Since the landmark FDA approval at the end of 2014 of the anti-CD3 × anti-CD19 bispecific antibody blinatumomab (Blincyto®) for the treatment of relapsed/refractory B-cell acute lymphoblastic leukaemia, ~100 clinical trials investigating the safety and efficacy of CD3+ bispecific T-cell redirectors for cancer have been initiated. However, despite early success, numerous challenges pertaining to CD3+ T-cell redirection in the context of cancer exist, including the recruitment of counterproductive CD3+ T-cell subsets, the release of systemic cytokines, the expansion of immune checkpoint molecules, the presence of an immunosuppressive tumour microenvironment, tumour antigen loss/escape, on-target off-tumour toxicity and suboptimal potency. The aim of the present review is to discuss novel approaches to overcome the key challenges associated with CD3+ bispecific T-cell redirection in order to achieve an optimal balance of anti-tumour activity and safety.
Conflict of interest statement
S.D. and I.S.G. are paid employees of the Janssen Pharmaceutical Companies of Johnson & Johnson and receive salary and other compensation.
Figures

References
-
- Goebeler ME, Bargou RC. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 2020;17:418–434. - PubMed
-
- Deshaies RJ. Multispecific drugs herald a new era of biopharmaceutical innovation. Nature. 2020;580:329–338. - PubMed
-
- Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019;18:585–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

