Development and therapeutic potential of 2-aminothiazole derivatives in anticancer drug discovery
- PMID: 33469255
- PMCID: PMC7809097
- DOI: 10.1007/s00044-020-02686-2
Development and therapeutic potential of 2-aminothiazole derivatives in anticancer drug discovery
Abstract
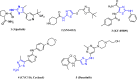
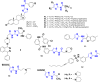
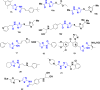
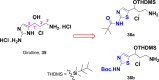
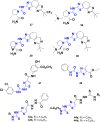
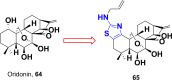
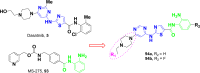
Currently, the development of anticancer drug resistance is significantly restricted the clinical efficacy of the most commonly prescribed anticancer drug. Malignant disease is widely prevalent and considered to be the major challenges of this century, which concerns the medical community all over the world. Consequently, investigating small molecule antitumor agents, which could decrease drug resistance and reduce unpleasant side effect is more desirable. 2-aminothiazole scaffold has emerged as a promising scaffold in medicinal chemistry and drug discovery research. This nucleus is a fundamental part of some clinically applied anticancer drugs such as dasatinib and alpelisib. Literature survey documented that different 2-aminothiazole analogs exhibited their potent and selective nanomolar inhibitory activity against a wide range of human cancerous cell lines such as breast, leukemia, lung, colon, CNS, melanoma, ovarian, renal, and prostate. In this paper, we have reviewed the progresses and structural modification of 2-aminothiazole to pursuit potent anticancers and also highlighted in vitro activities and in silico studies. The information will useful for future innovation. Representatives of 2-aminothiazole-containing compounds classification.
Keywords: 2-aminothiazole; Alpelisib; Anticancer; Dasatinib; Hantzsch’s synthesis; SAR study.
© The Author(s), under exclusive licence to Springer Science+Business Media, LLC part of Springer Nature 2021.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures







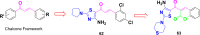







References
-
- WO Foye, TL Lemke, DA Williams, Principles of medicinal chemistry, Forth edn., PA: Williams and Wilkins, Media; 2002. p. 822.
-
- Stewart BW, Wild CP. World cancer report 2014. Lyon, France: International Agency for Research on Cancer (IARC); 2015.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
