CRNDE Contributes Cervical Cancer Progression by Regulating miR-4262/ZEB1 Axis
- PMID: 33469312
- PMCID: PMC7812045
- DOI: 10.2147/OTT.S263505
CRNDE Contributes Cervical Cancer Progression by Regulating miR-4262/ZEB1 Axis
Abstract
Background: Cervical cancer is a lethal gynecologic cancer in women. Long non-coding RNA colorectal neoplasia differentially expressed (LncRNA CRNDE) was recognized as a significant oncogene in multiple cancers. However, the functional role of CRNDE in cervical cancer remains poorly explored.
Methods: The expression of CRNDE, microRNA-4262 (miR-4262) and zinc-finger E-box binding homeobox 1 (ZEB1) in cervical cancer tumors and cells was detected by quantitative real-time polymerase chain reaction (qRT-PCR). Colony formation and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) were performed to detect cell viability. Flow cytometry and caspase-3 activity assay were conducted to evaluate cell apoptosis. The interaction between miR-4262 and CRNDE or ZEB1 was verified by dual-luciferase reporter system. Transwell assay was employed to evaluate cell migration and invasion. The relative protein expression was assessed by Western blot.
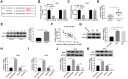
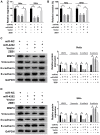
Results: CRNDE and ZEB1 were up-regulated, while miR-4262 was down-regulated in cervical cancer tissues and cells. We found that CRNDE sponged miR-4262 and ZEB1 was a target of miR-4262. In addition, miR-4262 inhibitor abolished CRNDE silencing-induced repression on cell proliferation, EMT, migration, invasion and promotion on cell apoptosis. Furthermore, ZEB1 rescued the effects of miR-4262 overexpression or CRNDE deletion on cervical cancer progression. Our data showed that CRNDE targeted miR-4262 to regulate ZEB1 expression in cervical cancer cells. Besides, CRNDE expedited cervical cancer progression through wnt/β-catenin pathway via sponging miR-4262 and altering ZEB1 expression.
Conclusion: Our findings demonstrated that CRNDE facilitated the progression of cervical cancer through activation of wnt/β-catenin pathway by regulating miR-4262/ZEB1 axis, representing a prospective targeted therapy for cervical cancer.
Keywords: CRNDE; ZEB1; cervical cancer; miR-4262; wnt/β-catenin pathway.
© 2021 Ren et al.
Conflict of interest statement
The authors declare that they have no financial conflicts of interest.
Figures





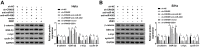
Similar articles
-
The Long Non-Coding RNA CRNDE Promotes Colorectal Carcinoma Progression by Competitively Binding miR-217 with TCF7L2 and Enhancing the Wnt/β-Catenin Signaling Pathway.Cell Physiol Biochem. 2017;41(6):2489-2502. doi: 10.1159/000475941. Epub 2017 May 4. Cell Physiol Biochem. 2017. PMID: 28472810
-
LncRNA PCAT6 Accelerates the Progression and Chemoresistance of Cervical Cancer Through Up-Regulating ZEB1 by Sponging miR-543.Onco Targets Ther. 2020 Feb 7;13:1159-1170. doi: 10.2147/OTT.S232354. eCollection 2020. Onco Targets Ther. 2020. PMID: 32103984 Free PMC article.
-
LncRNA CRNDE acts as an oncogene in cervical cancer through sponging miR-183 to regulate CCNB1 expression.Carcinogenesis. 2020 Mar 13;41(1):111-121. doi: 10.1093/carcin/bgz166. Carcinogenesis. 2020. PMID: 31605132
-
lncRNA CRNDE promotes the proliferation and metastasis by acting as sponge miR-539-5p to regulate POU2F1 expression in HCC.BMC Cancer. 2020 Apr 6;20(1):282. doi: 10.1186/s12885-020-06771-y. BMC Cancer. 2020. PMID: 32252678 Free PMC article.
-
The long non-coding RNA CRNDE promotes osteosarcoma proliferation and migration by sponging miR-136-5p/MRP9 axis.Ann Transl Med. 2022 Aug;10(15):835. doi: 10.21037/atm-22-3602. Ann Transl Med. 2022. PMID: 36034978 Free PMC article.
Cited by
-
CRNDE: A Pivotal Oncogenic Long Non-Coding RNA in Cancers.Yale J Biol Med. 2023 Dec 29;96(4):511-526. doi: 10.59249/VHYE2306. eCollection 2023 Dec. Yale J Biol Med. 2023. PMID: 38161583 Free PMC article. Review.
-
Serum exosomal long noncoding RNA CRNDE as a prognostic biomarker for hepatocellular carcinoma.J Clin Lab Anal. 2021 Nov;35(11):e23959. doi: 10.1002/jcla.23959. Epub 2021 Oct 6. J Clin Lab Anal. 2021. PMID: 34612554 Free PMC article.
-
Salidroside overcomes cisplatin resistance in ovarian cancer via the inhibition of CRNDE-mediated autophagy.Mol Cell Biochem. 2025 May;480(5):3097-3116. doi: 10.1007/s11010-024-05168-w. Epub 2024 Dec 5. Mol Cell Biochem. 2025. PMID: 39636431
-
Epigenetic and Transcriptomic Regulation Landscape in HPV+ Cancers: Biological and Clinical Implications.Front Genet. 2022 Jun 14;13:886613. doi: 10.3389/fgene.2022.886613. eCollection 2022. Front Genet. 2022. PMID: 35774512 Free PMC article. Review.
-
Analysis of the Role and Mechanism of ZEB1 in Regulating Cervical Carcinoma Progression via Modulating PD-1/PD-L1 Checkpoint.Comput Math Methods Med. 2022 Apr 30;2022:1565094. doi: 10.1155/2022/1565094. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Sep 27;2023:9783548. doi: 10.1155/2023/9783548. PMID: 35535226 Free PMC article. Retracted.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

