Acute and Long-Term Circuit-Level Effects in the Auditory Cortex After Sound Trauma
- PMID: 33469416
- PMCID: PMC7813782
- DOI: 10.3389/fnins.2020.598406
Acute and Long-Term Circuit-Level Effects in the Auditory Cortex After Sound Trauma
Abstract
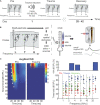
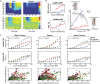
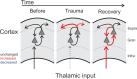
Harmful environmental sounds are a prevailing source of chronic hearing impairments, including noise induced hearing loss, hyperacusis, or tinnitus. How these symptoms are related to pathophysiological damage to the sensory receptor epithelia and its effects along the auditory pathway, have been documented in numerous studies. An open question concerns the temporal evolution of maladaptive changes after damage and their manifestation in the balance of thalamocortical and corticocortical input to the auditory cortex (ACx). To address these issues, we investigated the loci of plastic reorganizations across the tonotopic axis of the auditory cortex of male Mongolian gerbils (Meriones unguiculatus) acutely after a sound trauma and after several weeks. We used a residual current-source density analysis to dissociate adaptations of intracolumnar input and horizontally relayed corticocortical input to synaptic populations across cortical layers in ACx. A pure tone-based sound trauma caused acute changes of subcortical inputs and corticocortical inputs at all tonotopic regions, particularly showing a broad reduction of tone-evoked inputs at tonotopic regions around the trauma frequency. At other cortical sites, the overall columnar activity acutely decreased, while relative contributions of lateral corticocortical inputs increased. After 4-6 weeks, cortical activity in response to the altered sensory inputs showed a general increase of local thalamocortical input reaching levels higher than before the trauma. Hence, our results suggest a detailed mechanism for overcompensation of altered frequency input in the auditory cortex that relies on a changing balance of thalamocortical and intracortical input and along the frequency gradient of the cortical tonotopic map.
Keywords: auditory cortex; circuit level analysis; corticocortical; noise-induced hearing loss; thalamocortical.
Copyright © 2021 Jeschke, Happel, Tziridis, Krauss, Schilling, Schulze and Ohl.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Compensating Level-Dependent Frequency Representation in Auditory Cortex by Synaptic Integration of Corticocortical Input.PLoS One. 2017 Jan 3;12(1):e0169461. doi: 10.1371/journal.pone.0169461. eCollection 2017. PLoS One. 2017. PMID: 28046062 Free PMC article.
-
The effects of acute and chronic noise trauma on stimulus-evoked activity across primary auditory cortex layers.J Neurophysiol. 2024 Feb 1;131(2):225-240. doi: 10.1152/jn.00427.2022. Epub 2024 Jan 10. J Neurophysiol. 2024. PMID: 38198658
-
Optogenetic stimulation of the VTA modulates a frequency-specific gain of thalamocortical inputs in infragranular layers of the auditory cortex.Sci Rep. 2019 Dec 31;9(1):20385. doi: 10.1038/s41598-019-56926-6. Sci Rep. 2019. PMID: 31892726 Free PMC article.
-
Effects of long-term non-traumatic noise exposure on the adult central auditory system. Hearing problems without hearing loss.Hear Res. 2017 Sep;352:12-22. doi: 10.1016/j.heares.2016.10.015. Epub 2016 Oct 26. Hear Res. 2017. PMID: 27793584 Review.
-
Auditory thalamic circuits and GABAA receptor function: Putative mechanisms in tinnitus pathology.Hear Res. 2017 Jun;349:197-207. doi: 10.1016/j.heares.2016.08.009. Epub 2016 Aug 21. Hear Res. 2017. PMID: 27553899 Free PMC article. Review.
Cited by
-
Intrinsic Noise Improves Speech Recognition in a Computational Model of the Auditory Pathway.Front Neurosci. 2022 Jun 8;16:908330. doi: 10.3389/fnins.2022.908330. eCollection 2022. Front Neurosci. 2022. PMID: 35757533 Free PMC article.
-
Long-Term Moderate-Level Noise Exposure Caused Hyperexcitability in the Central Auditory System.Neural Plast. 2025 Jan 24;2025:8842073. doi: 10.1155/np/8842073. eCollection 2025. Neural Plast. 2025. PMID: 39949836 Free PMC article.
-
Stress Response and Hearing Loss Differentially Contribute to Dynamic Alterations in Hippocampal Neurogenesis and Microglial Reactivity in Mice Exposed to Acute Noise Exposure.Front Neurosci. 2021 Dec 8;15:749925. doi: 10.3389/fnins.2021.749925. eCollection 2021. Front Neurosci. 2021. PMID: 34955715 Free PMC article.
-
Cortical evoked activity is modulated by the sleep state in a ferret model of tinnitus. A cross-case study.PLoS One. 2024 Dec 4;19(12):e0304306. doi: 10.1371/journal.pone.0304306. eCollection 2024. PLoS One. 2024. PMID: 39630799 Free PMC article.
-
Development of Tinnitus and Hyperacusis in a Mouse Model of Tobramycin Cochleotoxicity.Front Mol Neurosci. 2021 Sep 1;14:715952. doi: 10.3389/fnmol.2021.715952. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34539342 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

