Calcium Sensors STIM1 and STIM2 Regulate Different Calcium Functions in Cultured Hippocampal Neurons
- PMID: 33469426
- PMCID: PMC7813759
- DOI: 10.3389/fnsyn.2020.573714
Calcium Sensors STIM1 and STIM2 Regulate Different Calcium Functions in Cultured Hippocampal Neurons
Abstract
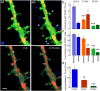
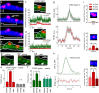
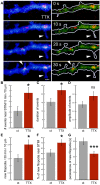
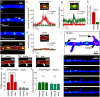
There are growing indications for the involvement of calcium stores in the plastic properties of neurons and particularly in dendritic spines of central neurons. The store-operated calcium entry (SOCE) channels are assumed to be activated by the calcium sensor stromal interaction molecule (STIM)which leads to activation of its associated Orai channel. There are two STIM species, and the differential role of the two in SOCE is not entirely clear. In the present study, we were able to distinguish between transfected STIM1, which is more mobile primarily in young neurons, and STIM2 which is less mobile and more prominent in older neurons in culture. STIM1 mobility is associated with spontaneous calcium sparks, local transient rise in cytosolic [Ca2+]i, and in the formation and elongation of dendritic filopodia/spines. In contrast, STIM2 is associated with older neurons, where it is mobile and moves into dendritic spines primarily when cytosolic [Ca2+]i levels are reduced, apparently to activate resident Orai channels. These results highlight a role for STIM1 in the regulation of [Ca2+]i fluctuations associated with the formation of dendritic spines or filopodia in the developing neuron, whereas STIM2 is associated with the maintenance of calcium entry into stores in the adult neuron.
Keywords: STIM; calcium stores; cytosolic calcium; dendritic spines; filopodia; hippocampal culture; store operated channels.
Copyright © 2021 Kushnireva, Korkotian and Segal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Hippocampal dendritic spines store-operated calcium entry and endoplasmic reticulum content is dynamic microtubule dependent.Sci Rep. 2025 Jan 8;15(1):1314. doi: 10.1038/s41598-024-85024-5. Sci Rep. 2025. PMID: 39779788 Free PMC article.
-
The role of the store-operated calcium entry channel Orai1 in cultured rat hippocampal synapse formation and plasticity.J Physiol. 2017 Jan 1;595(1):125-140. doi: 10.1113/JP272645. Epub 2016 Aug 8. J Physiol. 2017. PMID: 27393042 Free PMC article.
-
Cross-talk between N-terminal and C-terminal domains in stromal interaction molecule 2 (STIM2) determines enhanced STIM2 sensitivity.J Biol Chem. 2019 Apr 19;294(16):6318-6332. doi: 10.1074/jbc.RA118.006801. Epub 2019 Mar 1. J Biol Chem. 2019. PMID: 30824535 Free PMC article.
-
STIM and ORAI proteins in the nervous system.Channels (Austin). 2015;9(5):245-52. doi: 10.1080/19336950.2015.1071747. Epub 2015 Jul 28. Channels (Austin). 2015. PMID: 26218135 Free PMC article. Review.
-
STIM and Orai1 Variants in Store-Operated Calcium Entry.Front Pharmacol. 2016 Jan 13;6:325. doi: 10.3389/fphar.2015.00325. eCollection 2015. Front Pharmacol. 2016. PMID: 26793113 Free PMC article. Review.
Cited by
-
Gene-educational attainment interactions in a multi-population genome-wide meta-analysis identify novel lipid loci.Front Genet. 2023 Nov 2;14:1235337. doi: 10.3389/fgene.2023.1235337. eCollection 2023. Front Genet. 2023. PMID: 38028628 Free PMC article.
-
Alzheimer's Disease beyond Calcium Dysregulation: The Complex Interplay between Calmodulin, Calmodulin-Binding Proteins and Amyloid Beta from Disease Onset through Progression.Curr Issues Mol Biol. 2023 Jul 27;45(8):6246-6261. doi: 10.3390/cimb45080393. Curr Issues Mol Biol. 2023. PMID: 37623212 Free PMC article. Review.
-
Hippocampal dendritic spines store-operated calcium entry and endoplasmic reticulum content is dynamic microtubule dependent.Sci Rep. 2025 Jan 8;15(1):1314. doi: 10.1038/s41598-024-85024-5. Sci Rep. 2025. PMID: 39779788 Free PMC article.
-
SOCE as a regulator of neuronal activity.J Physiol. 2024 Apr;602(8):1449-1462. doi: 10.1113/JP283826. Epub 2023 Apr 22. J Physiol. 2024. PMID: 37029630 Free PMC article. Review.
-
Presynaptic store-operated Ca2+ entry drives excitatory spontaneous neurotransmission and augments endoplasmic reticulum stress.Neuron. 2021 Apr 21;109(8):1314-1332.e5. doi: 10.1016/j.neuron.2021.02.023. Epub 2021 Mar 11. Neuron. 2021. PMID: 33711258 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

