This is a preprint.
Tropism of SARS-CoV-2 for Developing Human Cortical Astrocytes
- PMID: 33469577
- PMCID: PMC7814814
- DOI: 10.1101/2021.01.17.427024
Tropism of SARS-CoV-2 for Developing Human Cortical Astrocytes
Update in
-
Tropism of SARS-CoV-2 for human cortical astrocytes.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2122236119. doi: 10.1073/pnas.2122236119. Epub 2022 Jul 12. Proc Natl Acad Sci U S A. 2022. PMID: 35858406 Free PMC article.
Abstract
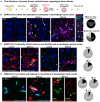
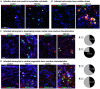
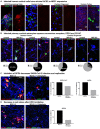
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) readily infects a variety of cell types impacting the function of vital organ systems, with particularly severe impact on respiratory function. It proves fatal for one percent of those infected. Neurological symptoms, which range in severity, accompany a significant proportion of COVID-19 cases, indicating a potential vulnerability of neural cell types. To assess whether human cortical cells can be directly infected by SARS-CoV-2, we utilized primary human cortical tissue and stem cell-derived cortical organoids. We find significant and predominant infection in cortical astrocytes in both primary and organoid cultures, with minimal infection of other cortical populations. Infected astrocytes had a corresponding increase in reactivity characteristics, growth factor signaling, and cellular stress. Although human cortical cells, including astrocytes, have minimal ACE2 expression, we find high levels of alternative coronavirus receptors in infected astrocytes, including DPP4 and CD147. Inhibition of DPP4 reduced infection and decreased expression of the cell stress marker, ARCN1. We find tropism of SARS-CoV-2 for human astrocytes mediated by DPP4, resulting in reactive gliosis-type injury.
Figures

References
-
- Varatharaj A., Thomas N., Ellul M. A., Davies N. W. S., Pollak T. A., Tenorio E. L., Sultan M., Easton A., Breen G., Zandi M., Coles J. P., Manji H., Al-Shahi Salman R., Menon D. K., Nicholson T. R., Benjamin L. A., Carson A., Smith C., Turner M. R., Solomon T., Kneen R., Pett S. L., Galea I., Thomas R. H., Michael B. D., CoroNerve Study Group, Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 7, 875–882 (2020). - PMC - PubMed
-
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., Lehmann M., Hassan O., Aschman T., Schumann E., Chua R. L., Conrad C., Eils R., Stenzel W., Windgassen M., Rößler L., Goebel H.-H., Gelderblom H. R., Martin H., Nitsche A., Schulz-Schaeffer W. J., Hakroush S., Winkler M. S., Tampe B., Scheibe F., Körtvélyessy P., Reinhold D., Siegmund B., Kühl A. A., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold-Heppner B., Stadelmann C., Drosten C., Corman V. M., Radbruch H., Heppner F. L., Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. (2020), doi: 10.1038/s41593-020-00758-5. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
