The solution structures of higher-order human telomere G-quadruplex multimers
- PMID: 33469644
- PMCID: PMC7897503
- DOI: 10.1093/nar/gkaa1285
The solution structures of higher-order human telomere G-quadruplex multimers
Abstract
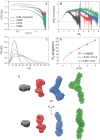
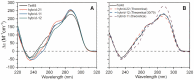
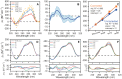
Human telomeres contain the repeat DNA sequence 5'-d(TTAGGG), with duplex regions that are several kilobases long terminating in a 3' single-stranded overhang. The structure of the single-stranded overhang is not known with certainty, with disparate models proposed in the literature. We report here the results of an integrated structural biology approach that combines small-angle X-ray scattering, circular dichroism (CD), analytical ultracentrifugation, size-exclusion column chromatography and molecular dynamics simulations that provide the most detailed characterization to date of the structure of the telomeric overhang. We find that the single-stranded sequences 5'-d(TTAGGG)n, with n = 8, 12 and 16, fold into multimeric structures containing the maximal number (2, 3 and 4, respectively) of contiguous G4 units with no long gaps between units. The G4 units are a mixture of hybrid-1 and hybrid-2 conformers. In the multimeric structures, G4 units interact, at least transiently, at the interfaces between units to produce distinctive CD signatures. Global fitting of our hydrodynamic and scattering data to a worm-like chain (WLC) model indicates that these multimeric G4 structures are semi-flexible, with a persistence length of ∼34 Å. Investigations of its flexibility using MD simulations reveal stacking, unstacking, and coiling movements, which yield unique sites for drug targeting.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

