Metabolomic profiling of rare cell populations isolated by flow cytometry from tissues
- PMID: 33470192
- PMCID: PMC7847306
- DOI: 10.7554/eLife.61980
Metabolomic profiling of rare cell populations isolated by flow cytometry from tissues
Abstract
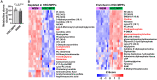
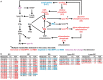
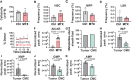
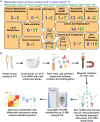
Little is known about the metabolic regulation of rare cell populations because most metabolites are hard to detect in small numbers of cells. We previously described a method for metabolomic profiling of flow cytometrically isolated hematopoietic stem cells (HSCs) that detects 60 metabolites in 10,000 cells (Agathocleous et al., 2017). Here we describe a new method involving hydrophilic liquid interaction chromatography and high-sensitivity orbitrap mass spectrometry that detected 160 metabolites in 10,000 HSCs, including many more glycolytic and lipid intermediates. We improved chromatographic separation, increased mass resolution, minimized ion suppression, and eliminated sample drying. Most metabolite levels did not significantly change during cell isolation. Mouse HSCs exhibited increased glycerophospholipids relative to bone marrow cells and methotrexate treatment altered purine biosynthesis. Circulating human melanoma cells were depleted for purine intermediates relative to subcutaneous tumors, suggesting decreased purine synthesis during metastasis. These methods facilitate the routine metabolomic analysis of rare cells from tissues.
Keywords: circulating cancer cell; hematopoietic stem cell; melanoma; metabolism; metabolomics; mouse; regenerative medicine; stem cells.
© 2021, DeVilbiss et al.
Conflict of interest statement
AD, ZZ, MM, JU, AT, MA, TM No competing interests declared, SM advisor for Frequency Therapeutics and Protein Fluidics as well as a stockholder in G1 Therapeutics.
Figures





References
-
- Abouleila Y, Onidani K, Ali A, Shoji H, Kawai T, Lim CT, Kumar V, Okaya S, Kato K, Hiyama E, Yanagida T, Masujima T, Shimizu Y, Honda K. Live single cell mass spectrometry reveals cancer-specific metabolic profiles of circulating tumor cells. Cancer Science. 2019;110:697–706. doi: 10.1111/cas.13915. - DOI - PMC - PubMed
-
- Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, Spangrude GJ, Hu Z, DeBerardinis RJ, Morrison SJ. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549:476–481. doi: 10.1038/nature23876. - DOI - PMC - PubMed
-
- Ali A, Abouleila Y, Shimizu Y, Hiyama E, Emara S, Mashaghi A, Hankemeier T. Single-cell metabolomics by mass spectrometry: advances, challenges, and future applications. TrAC Trends in Analytical Chemistry. 2019;120:115436. doi: 10.1016/j.trac.2019.02.033. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

