Crizotinib for recurring non-small-cell lung cancer with EML4-ALK fusion genes previously treated with alectinib: A phase II trial
- PMID: 33470536
- PMCID: PMC7919114
- DOI: 10.1111/1759-7714.13825
Crizotinib for recurring non-small-cell lung cancer with EML4-ALK fusion genes previously treated with alectinib: A phase II trial
Abstract
Background: The efficacy of crizotinib treatment for recurring EML4-ALK-positive non-small cell lung cancer (NSCLC) previously treated with alectinib is unclear. Based on our preclinical findings regarding hepatocyte growth factor/mesenchymal epithelial transition (MET) pathway activation as a potential mechanism of acquired resistance to alectinib, we conducted a phase II trial of the anaplastic lymphoma kinase/MET inhibitor, crizotinib, in patients with alectinib-refractory, EML4-ALK-positive NSCLC.
Methods: Patients with ALK-rearranged tumors treated with alectinib immediately before enrolling in the trial received crizotinib monotherapy. The objective response rate was the primary outcome of interest.
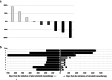
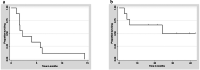
Results: Nine (100%) patients achieved a partial response with alectinib therapy with a median treatment duration of 6.7 months. Crizotinib was administered with a median treatment interval of 50 (range, 20-433) days. The overall response rate was 33.3% (90% confidence interval [CI]: 9.8-65.5 and 95% CI: 7.5-70.1), which did not reach the predefined criteria of 50%. Two (22%) patients who achieved a partial response had brain metastases at baseline. Progression-free survival (median, 2.2 months) was not affected by the duration of treatment with alectinib. The median survival time was 24.1 months. The most common adverse events were an increased aspartate transaminase/alanine transaminase (AST/ALT) ratio (44%) and appetite loss (33%); one patient developed transient grade 4 AST/ALT elevation, resulting in treatment discontinuation. Other adverse events were consistent with those previously reported; no treatment-related deaths occurred.
Conclusions: Although the desired response rate was not achieved, crizotinib monotherapy following treatment with alectinib showed efficacy alongside previously described adverse events.
Keywords: Alectinib; anaplastic lymphoma kinase; crizotinib; drug therapy; non-small cell lung carcinoma.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
This work received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. DH received research funds from Lilly, MSD, Chugai, Pfizer, BMS, AstraZeneca, Novartis, Kissei, and Takeda and lecture fees from MSD, Ono, BMS, Kyowa Hakko Kirin, AstraZeneca, Boehringer Ingelheim, and Lilly. TK received research funds from Chugai, AstraZeneca, Lilly, Taiho, BMS, and Merck and lecture fees from Chugai, AstraZeneca, Lilly, Taiho, BMS, Ono, MSD, Pfizer, Kyowa Hakko Kirin, Boehringer Ingelheim, Nippon Kayaku, and Novartis. TY received lecture fees from AstraZeneca, Boehringer Ingelheim, Lilly, MSD, Novartis, Pfizer, Ono, and Chugai. HY received lecture fees from AstraZeneca, Boehringer Ingelheim, Lilly, MSD, and Chugai. AB received research funds from Pfizer. NT received research funds from AstraZeneca, Daiichi‐Sankyo, Chugai, Taiho, Pfizer, Boehringer Ingelheim, Ono, MSD, Lilly, Kyowa Hakko Kirin, and Nippon Kayaku; lecture fees from AstraZeneca, Daiichi‐Sankyo, Boehringer Ingelheim, Taiho, Pfizer, Ono, MSD, Lilly, and Chugai; and scholarship endowments from Lilly, Boehringer Ingelheim, and Ono. KH received lecture fees from Pfizer, Lilly, AstraZeneca, BMS, Ono, MSD, Chugai, Nippon Kayaku, Taiho, Boehringer Ingelheim, Novartis, Daiichi‐Sankyo, and Kyorin and research funds from Chugai, Lilly, BMS, AstraZeneca, MSD, and Astellas. KK received lecture fees from Astellas, Ono, Taiho, Chugai, BMS, Pfizer, Novartis, Boehringer Ingelheim, and MSD; research funds from Boehringer Ingelheim, Taiho, Chugai, Nippon Kayaku, Ono, BMS, MSD, Pfizer, Teijin, Shionogi, and Kyorin; and scholarship endowments from Taiho, Boehringer Ingelheim, Nippon Kayaku, Ono, Chugai, and BMS. None of the other authors had any potential conflict of interest.
Figures
Similar articles
-
Protocol Design for the Bench to Bed Trial in Alectinib-Refractory Non-Small-Cell Lung Cancer Patients Harboring the EML4-ALK Fusion Gene (ALRIGHT/OLCSG1405).Clin Lung Cancer. 2016 Nov;17(6):602-605. doi: 10.1016/j.cllc.2016.05.005. Epub 2016 Jun 2. Clin Lung Cancer. 2016. PMID: 27405684 Clinical Trial.
-
Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study.Lancet Respir Med. 2019 May;7(5):437-446. doi: 10.1016/S2213-2600(19)30053-0. Epub 2019 Apr 10. Lancet Respir Med. 2019. PMID: 30981696 Clinical Trial.
-
Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study.Lancet Oncol. 2014 Sep;15(10):1119-28. doi: 10.1016/S1470-2045(14)70362-6. Epub 2014 Aug 18. Lancet Oncol. 2014. PMID: 25153538 Clinical Trial.
-
The use of alectinib in the first-line treatment of anaplastic lymphoma kinase-positive non-small-cell lung cancer.Future Oncol. 2018 Aug;14(18):1875-1882. doi: 10.2217/fon-2018-0027. Epub 2018 Mar 14. Future Oncol. 2018. PMID: 29536761 Review.
-
Alectinib: A Review in Advanced, ALK-Positive NSCLC.Drugs. 2018 Aug;78(12):1247-1257. doi: 10.1007/s40265-018-0952-0. Drugs. 2018. PMID: 30030733 Review.
Cited by
-
Current perspectives in drug targeting intrinsically disordered proteins and biomolecular condensates.BMC Biol. 2025 May 6;23(1):118. doi: 10.1186/s12915-025-02214-x. BMC Biol. 2025. PMID: 40325419 Free PMC article. Review.
-
Lck Function and Modulation: Immune Cytotoxic Response and Tumor Treatment More Than a Simple Event.Cancers (Basel). 2024 Jul 24;16(15):2630. doi: 10.3390/cancers16152630. Cancers (Basel). 2024. PMID: 39123358 Free PMC article. Review.
-
Fibroblast growth factor receptor 3 overexpression mediates ALK inhibitor resistance in ALK-rearranged non-small cell lung cancer.Cancer Sci. 2022 Nov;113(11):3888-3900. doi: 10.1111/cas.15529. Epub 2022 Sep 11. Cancer Sci. 2022. PMID: 35950895 Free PMC article.
-
Crizotinib inhibits the metabolism of tramadol by non-competitive suppressing the activities of CYP2D1 and CYP3A2.PeerJ. 2024 May 30;12:e17446. doi: 10.7717/peerj.17446. eCollection 2024. PeerJ. 2024. PMID: 38827306 Free PMC article.
-
MET gene amplification is a mechanism of resistance to entrectinib in ROS1+ NSCLC.Thorac Cancer. 2022 Nov;13(21):3032-3041. doi: 10.1111/1759-7714.14656. Epub 2022 Sep 13. Thorac Cancer. 2022. PMID: 36101520 Free PMC article.
References
-
- Isozaki H, Hotta K, Ichihara E, Takigawa N, Ohashi K, Kubo T, et al. Protocol design for the bench to bed trial in alectinib‐refractory non‐small‐cell lung cancer patients harboring the EML4‐ALK fusion gene (ALRIGHT/OLCSG1405). Clin Lung Cancer. 2016;17:602–5. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. - PubMed
-
- Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med. 2013;368:2385–94. - PubMed
-
- Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371:2167–77. - PubMed
-
- Nakagawa K, Hida T, Nokihara H, Morise M, Azuma K, Kim YH, et al. Final progression‐free survival results from the J‐ALEX study of alectinib versus crizotinib in ALK‐positive non‐small‐cell lung cancer. Lung Cancer. 2020;139:195–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

