Multifunctional Neomycin-Triazine-Based Cationic Lipids for Gene Delivery with Antibacterial Properties
- PMID: 33470802
- PMCID: PMC8154203
- DOI: 10.1021/acs.bioconjchem.0c00616
Multifunctional Neomycin-Triazine-Based Cationic Lipids for Gene Delivery with Antibacterial Properties
Abstract
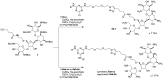
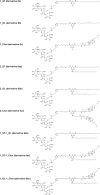
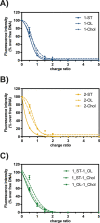
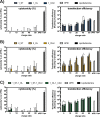
Cationic lipids (CLs) have gained significant attention among nonviral gene delivery vectors due to their ease of synthesis and functionalization with multivalent moieties. In particular, there is an increasing request for multifunctional CLs having gene delivery capacity and antibacterial activity. Herein, we describe the design and synthesis of a novel class of aminoglycoside (AG)-based multifunctional vectors with high transfection efficiency and noticeable antibacterial properties. Specifically, cationic amphiphiles were built on a triazine scaffold, allowing for an easy derivatization with up to three potentially different substituents, such as neomycin (Neo) that serves as the polar head and one or two lipophilic tails, namely stearyl (ST) and oleyl (OL) alkyl chains and cholesteryl (Chol) tail. With the aim to shed more light on the effect of different types and numbers of lipophilic moieties on the ability of CLs to condense and transfect cells, the performance of Neo-triazine-based derivatives as gene delivery vectors was evaluated and compared. The ability of Neo-triazine-based derivatives to act as antimicrobial agents was evaluated as well. Neo-triazine-based CLs invariably exhibited excellent DNA condensation ability, even at a low charge ratio (CR, +/-). Besides, each derivative showed very good transfection performance at its optimal CR on two different cell lines, along with negligible cytotoxicity. CLs bearing symmetric two-tailed OL proved to be the most effective in transfection. Interestingly, Neo-triazine-based derivatives, used as either free lipids or lipoplexes, exhibited strong antibacterial activity against Gram-negative bacteria, especially in the case of CLs bearing one or two aliphatic chains. Altogether, these results highlight the potential of Neo-triazine-based derivatives as effective multifunctional nonviral gene delivery vectors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Design and synthesis of biologically active cationic amphiphiles built on the calix[4]arene scaffold.Int J Pharm. 2018 Oct 5;549(1-2):436-445. doi: 10.1016/j.ijpharm.2018.08.020. Epub 2018 Aug 14. Int J Pharm. 2018. PMID: 30118833
-
Dimerizable redox-sensitive triazine-based cationic lipids for in vitro gene delivery.ChemMedChem. 2007 Mar;2(3):292-6. doi: 10.1002/cmdc.200600267. ChemMedChem. 2007. PMID: 17191293 No abstract available.
-
Paromomycin and neomycin B derived cationic lipids: synthesis and transfection studies.J Control Release. 2012 Mar 28;158(3):461-9. doi: 10.1016/j.jconrel.2011.12.019. Epub 2011 Dec 29. J Control Release. 2012. PMID: 22226775
-
Cationic lipids for gene delivery: many players, one goal.Chem Phys Lipids. 2021 Mar;235:105032. doi: 10.1016/j.chemphyslip.2020.105032. Epub 2021 Jan 8. Chem Phys Lipids. 2021. PMID: 33359210 Review.
-
The headgroup evolution of cationic lipids for gene delivery.Bioconjug Chem. 2013 Apr 17;24(4):487-519. doi: 10.1021/bc300381s. Epub 2013 Apr 4. Bioconjug Chem. 2013. PMID: 23461774 Review.
Cited by
-
Restoring susceptibility to aminoglycosides: identifying small molecule inhibitors of enzymatic inactivation.RSC Med Chem. 2023 Jul 21;14(9):1591-1602. doi: 10.1039/d3md00226h. eCollection 2023 Sep 19. RSC Med Chem. 2023. PMID: 37731693 Free PMC article. Review.
-
Effect of Methylation of the Hydrophilic Domain of Tocopheryl Ammonium-Based Lipids on their Nucleic Acid Delivery Properties.ACS Omega. 2022 Apr 29;7(18):15396-15403. doi: 10.1021/acsomega.1c06889. eCollection 2022 May 10. ACS Omega. 2022. PMID: 35571792 Free PMC article.
-
Approaches towards biomaterial-mediated gene editing for cancer immunotherapy.Biomater Sci. 2022 Nov 22;10(23):6675-6687. doi: 10.1039/d2bm00806h. Biomater Sci. 2022. PMID: 35858470 Free PMC article. Review.
-
Profiling patent compounds in lipid nanoparticle formulations of siRNA.Mol Ther Nucleic Acids. 2024 Oct 18;35(4):102362. doi: 10.1016/j.omtn.2024.102362. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39554995 Free PMC article.
-
In vivo assessment of triazine lipid nanoparticles as transfection agents for plasmid DNA.Biomater Sci. 2022 Dec 6;10(24):6968-6979. doi: 10.1039/d2bm01289h. Biomater Sci. 2022. PMID: 36222485 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

