Economic burden of peanut allergy in pediatric patients with evidence of reactions to peanuts in the United States
- PMID: 33470880
- PMCID: PMC10394212
- DOI: 10.18553/jmcp.2021.20389
Economic burden of peanut allergy in pediatric patients with evidence of reactions to peanuts in the United States
Abstract
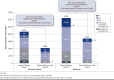
BACKGROUND: The economic burden of food allergy is large; however, costs specific to individuals with peanut allergy experiencing reactions to peanuts remain to be evaluated. As the prevalence of peanut allergy continues to increase in children, a better understanding of the cost of care is warranted. OBJECTIVE: To assess the cost of care of peanut allergy among privately insured and Medicaid-insured pediatric patients in the United States. METHODS: This retrospective matched-cohort study included patients aged 4-17 years from the Optum Health Care Solutions and Medicaid Claims databases (January 1, 2007-March 31, 2017). Patients were classified into 2 cohorts: peanut allergy (with peanut allergy diagnosis codes and reactions triggering health care resource utilization [HRU]) and peanut allergy-free (no peanut allergy diagnosis codes in claims). Peanut allergy patients were matched 1:10 to peanut allergy-free patients based on baseline covariates. Comorbidities including anxiety and depression, HRU, and direct health care costs were compared between cohorts and reported for both perspectives separately. RESULTS: Compared with peanut allergy-free patients (n = 30,840 privately insured; n = 12,450 Medicaid), peanut allergy patients (n = 3,084 privately insured; n = 1,245 Medicaid) had higher prevalence of asthma, atopic dermatitis/eczema, other food allergies, allergic rhinitis, depression, and anxiety (all P < 0.01). Peanut allergy patients had higher HRU per patient per year (PPPY), including 90% more emergency department visits among both privately insured and Medicaid patients (P < 0.01) and higher direct health care costs PPPY, with incremental costs of $2,247 total or $1,712 excluding asthma-related costs for privately insured patients and $2,845 total or $1,844 excluding asthma-related costs for Medicaid patients (all P < 0.01). CONCLUSIONS: Pediatric patients in the United States with peanut allergy and reactions triggering HRU had significantly higher comorbidity burdens, HRU, and direct health care costs, regardless of asthma-related costs, versus those without peanut allergy. DISCLOSURES: This study was funded by Aimmune Therapeutics, a Nestlé Health Science company. The study sponsor was involved in several aspects of the research including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication. Yu and Tilles are employees of Aimmune Therapeutics, a Nestlé Health Science company. Robison and Norrett were employees of Aimmune Therapeutics at the time this study was conducted. Blaiss, Meadows, and Hass provided paid consulting services to Aimmune Therapeutics. Guerin and Latremouille-Viau are employees of Analysis Group, a consulting company that provided paid consulting services to Aimmune Therapeutics. Parts of the results were presented at the AMCP Managed Care & Specialty Pharmacy Annual Meeting held March 25-28, 2019, in San Diego, CA, and at the ISPOR Annual Meeting held May 18-22, 2019, in New Orleans, LA.
Conflict of interest statement
This study was funded by Aimmune Therapeutics, a Nestlé Health Science company. The study sponsor was involved in several aspects of the research including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication. Yu and Tilles are employees of Aimmune Therapeutics, a Nestlé Health Science company. Robison and Norrett were employees of Aimmune Therapeutics at the time this study was conducted. Blaiss, Meadows, and Hass provided paid consulting services to Aimmune Therapeutics. Guerin and Latremouille-Viau are employees of Analysis Group, a consulting company that provided paid consulting services to Aimmune Therapeutics.
Parts of the results were presented at the AMCP Managed Care & Specialty Pharmacy Annual Meeting held March 25-28, 2019, in San Diego, CA, and at the ISPOR Annual Meeting held May 18-22, 2019, in New Orleans, LA.
Figures

Similar articles
-
Economic Burden of Treatment-Resistant Depression in Privately Insured U.S. Patients with Physical Conditions.J Manag Care Spec Pharm. 2020 Aug;26(8):996-1007. doi: 10.18553/jmcp.2020.20017. Epub 2020 Jun 19. J Manag Care Spec Pharm. 2020. PMID: 32552362 Free PMC article.
-
Health-care resource utilization associated with peanut allergy management under allergen avoidance among commercially insured individuals.Allergy Asthma Proc. 2021 Jul 1;42(4):333-342. doi: 10.2500/aap.2021.42.210047. Allergy Asthma Proc. 2021. PMID: 34187625
-
Economic and Clinical Burden of Acute Myeloid Leukemia Episodes of Care in the United States: A Retrospective Analysis of a Commercial Payer Database.J Manag Care Spec Pharm. 2020 Jul;26(7):849-859. doi: 10.18553/jmcp.2020.19220. Epub 2020 Apr 13. J Manag Care Spec Pharm. 2020. PMID: 32281456 Free PMC article.
-
Peanut (Arachis hypogaea) allergen powder-dnfp for the mitigation of allergic reactions to peanuts in children and adolescents.Expert Rev Clin Immunol. 2023 Mar;19(3):253-265. doi: 10.1080/1744666X.2023.2159812. Epub 2022 Dec 22. Expert Rev Clin Immunol. 2023. PMID: 36524617 Review.
-
A review of food allergy-related costs with consideration to clinical and demographic factors.Curr Opin Allergy Clin Immunol. 2023 Jun 1;23(3):246-251. doi: 10.1097/ACI.0000000000000903. Epub 2023 Mar 28. Curr Opin Allergy Clin Immunol. 2023. PMID: 37185830 Review.
Cited by
-
Children and caregiver proxy quality of life from peanut oral immunotherapy trials.Clin Transl Allergy. 2022 Dec;12(12):e12213. doi: 10.1002/clt2.12213. Clin Transl Allergy. 2022. PMID: 36573312 Free PMC article.
-
White paper on peanut allergy - part 1: Epidemiology, burden of disease, health economic aspects.Allergo J Int. 2021;30(8):261-269. doi: 10.1007/s40629-021-00189-z. Epub 2021 Sep 28. Allergo J Int. 2021. PMID: 34603938 Free PMC article.
-
Diagnosis of Peanut Allergy in Preschool Children: The Impact of Skin Testing With a Novel Composition of Peanuts.Front Pediatr. 2021 Nov 30;9:739224. doi: 10.3389/fped.2021.739224. eCollection 2021. Front Pediatr. 2021. PMID: 34917557 Free PMC article.
-
Evaluation and Modification of a Shared Decision-Making Tool for Peanut Allergy Management.Curr Allergy Asthma Rep. 2024 Jun;24(6):303-315. doi: 10.1007/s11882-024-01146-w. Epub 2024 Apr 19. Curr Allergy Asthma Rep. 2024. PMID: 38639896 Review.
-
Advances in Shellfish Allergy Therapy: From Current Approaches to Future Strategies.Clin Rev Allergy Immunol. 2025 Jul 16;68(1):65. doi: 10.1007/s12016-025-09077-8. Clin Rev Allergy Immunol. 2025. PMID: 40668267 Free PMC article. Review.
References
-
- Gupta RS, Springston EE, Warrier MR, et al. . The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9-17. - PubMed
-
- Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA.. U.S. prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322-26. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

