Chitinase 3 like 1 is a regulator of smooth muscle cell physiology and atherosclerotic lesion stability
- PMID: 33471078
- PMCID: PMC8848327
- DOI: 10.1093/cvr/cvab014
Chitinase 3 like 1 is a regulator of smooth muscle cell physiology and atherosclerotic lesion stability
Abstract
Aims: Atherosclerotic cerebrovascular disease underlies the majority of ischaemic strokes and is a major cause of death and disability. While plaque burden is a predictor of adverse outcomes, plaque vulnerability is increasingly recognized as a driver of lesion rupture and risk for clinical events. Defining the molecular regulators of carotid instability could inform the development of new biomarkers and/or translational targets for at-risk individuals.
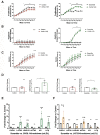
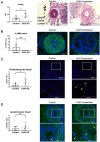
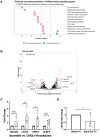
Methods and results: Using two independent human endarterectomy biobanks, we found that the understudied glycoprotein, chitinase 3 like 1 (CHI3L1), is up-regulated in patients with carotid disease compared to healthy controls. Further, CHI3L1 levels were found to stratify individuals based on symptomatology and histopathological evidence of an unstable fibrous cap. Gain- and loss-of-function studies in cultured human carotid artery smooth muscle cells (SMCs) showed that CHI3L1 prevents a number of maladaptive changes in that cell type, including phenotype switching towards a synthetic and hyperproliferative state. Using two murine models of carotid remodelling and lesion vulnerability, we found that knockdown of Chil1 resulted in larger neointimal lesions comprised by de-differentiated SMCs that failed to invest within and stabilize the fibrous cap. Exploratory mechanistic studies identified alterations in potential downstream regulatory genes, including large tumour suppressor kinase 2 (LATS2), which mediates macrophage marker and inflammatory cytokine expression on SMCs, and may explain how CHI3L1 modulates cellular plasticity.
Conclusion: CHI3L1 is up-regulated in humans with carotid artery disease and appears to be a strong mediator of plaque vulnerability. Mechanistic studies suggest this change may be a context-dependent adaptive response meant to maintain vascular SMCs in a differentiated state and to prevent rupture of the fibrous cap. Part of this effect may be mediated through downstream suppression of LATS2. Future studies should determine how these changes occur at the molecular level, and whether this gene can be targeted as a novel translational therapy for subjects at risk of stroke.
Keywords: CHI3L1; Carotid stenosis; Dedifferentiation; Stroke; Vascular smooth muscle cells; Vulnerable plaque.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures


Comment in
-
Vascular smooth muscle cell phenotypic switching and plaque stability: a role for CHI3L1.Cardiovasc Res. 2021 Dec 17;117(14):2691-2693. doi: 10.1093/cvr/cvab099. Cardiovasc Res. 2021. PMID: 33757119 Free PMC article. No abstract available.
References
-
- World Health Organisation - Healthinfo on Global Burden Disease. https://www.who.int/healthinfo/global_burden_disease/GHE2016_Deaths_Glob... (6 February 2020, date last accessed).
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA.. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095–2128. - PMC - PubMed
-
- Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D, American Heart Association Stroke Council CoCNCoCC, Interdisciplinary Council on Quality of C, Outcomes R. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–276. - PubMed
-
- Libby P, Ridker PM, Hansson GK.. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–325. - PubMed
-
- Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A, Hamilton G, Kakisis J, Kakkos S, Lepidi S, Markus HS, McCabe DJ, Roy J, Sillesen H, van den Berg JC, Vermassen F, Esvs Guidelines C, Kolh P, Chakfe N, Hinchliffe RJ, Koncar I, Lindholt JS, Vega de Ceniga M, Verzini F, Esvs Guideline R, Archie J, Bellmunt S, Chaudhuri A, Koelemay M, Lindahl AK, Padberg F, Venermo M.. Editor's choice - management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55:3–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

