Pharmacological validation of TDO as a target for Parkinson's disease
- PMID: 33471408
- PMCID: PMC8359396
- DOI: 10.1111/febs.15721
Pharmacological validation of TDO as a target for Parkinson's disease
Abstract
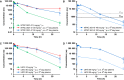
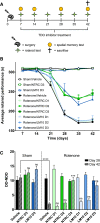
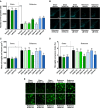
Parkinson's disease patients suffer from both motor and nonmotor impairments. There is currently no cure for Parkinson's disease, and the most commonly used treatment, levodopa, only functions as a temporary relief of motor symptoms. Inhibition of the expression of the L-tryptophan-catabolizing enzyme tryptophan 2,3-dioxygenase (TDO) has been shown to inhibit aging-related α-synuclein toxicity in Caenorhabditis elegans. To evaluate TDO inhibition as a potential therapeutic strategy for Parkinson's disease, a brain-penetrable, small molecule TDO inhibitor was developed, referred to as NTRC 3531-0. This compound potently inhibits human and mouse TDO in biochemical and cell-based assays and is selective over IDO1, an evolutionary unrelated enzyme that catalyzes the same reaction. In mice, NTRC 3531-0 increased plasma and brain L-tryptophan levels after oral administration, demonstrating inhibition of TDO activity in vivo. The effect on Parkinson's disease symptoms was evaluated in a rotenone-induced Parkinson's disease mouse model. A structurally dissimilar TDO inhibitor, LM10, was evaluated in parallel. Both inhibitors had beneficial effects on rotenone-induced motor and cognitive dysfunction as well as rotenone-induced dopaminergic cell loss and neuroinflammation in the substantia nigra. Moreover, both inhibitors improved intestinal transit and enhanced colon length, which indicates a reduction of the rotenone-induced intestinal dysfunction. Consistent with this, mice treated with TDO inhibitor showed decreased expression of rotenone-induced glial fibrillary acidic protein, which is a marker of enteric glial cells, and decreased α-synuclein accumulation in the enteric plexus. Our data support TDO inhibition as a potential therapeutic strategy to decrease motor, cognitive, and gastrointestinal symptoms in Parkinson's disease.
Keywords: L-tryptophan; blood-brain barrier; enzyme inhibitors; rotenone; tryptophan 2,3-dioxygenase.
© 2021 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
R.C. Buijsman and G.J.R. Zaman are managing directors and shareholders of Netherlands Translational Research Center B.V. The other authors have no potential conflicts of interest.
Figures




References
-
- Reich SG & Savitt JM (2019) Parkinson’s disease. Med Clin North Am 103, 337–350. - PubMed
-
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White III CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN & Walker DG & Arizona Parkinson’s Disease Consortium (2010) Multi‐organ distribution of phosphorylated alpha‐synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119, 689–702. - PMC - PubMed
-
- Poewe W (2008) Non‐motor symptoms in Parkinson’s disease. Eur J Neurol 15 (Suppl 1), 14–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

