Suitcase Lab for Rapid Detection of SARS-CoV-2 Based on Recombinase Polymerase Amplification Assay
- PMID: 33471510
- PMCID: PMC7839158
- DOI: 10.1021/acs.analchem.0c04779
Suitcase Lab for Rapid Detection of SARS-CoV-2 Based on Recombinase Polymerase Amplification Assay
Abstract
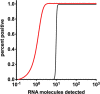
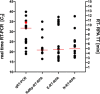
In March 2020, the SARS-CoV-2 virus outbreak was declared as a world pandemic by the World Health Organization (WHO). The only measures for controlling the outbreak are testing and isolation of infected cases. Molecular real-time polymerase chain reaction (PCR) assays are very sensitive but require highly equipped laboratories and well-trained personnel. In this study, a rapid point-of-need detection method was developed to detect the RNA-dependent RNA polymerase (RdRP), envelope protein (E), and nucleocapsid protein (N) genes of SARS-CoV-2 based on the reverse transcription recombinase polymerase amplification (RT-RPA) assay. RdRP, E, and N RT-RPA assays required approximately 15 min to amplify 2, 15, and 15 RNA molecules of molecular standard/reaction, respectively. RdRP and E RT-RPA assays detected SARS-CoV-1 and 2 genomic RNA, whereas the N RT-RPA assay identified only SARS-CoV-2 RNA. All established assays did not cross-react with nucleic acids of other respiratory pathogens. The RT-RPA assay's clinical sensitivity and specificity in comparison to real-time RT-PCR (n = 36) were 94 and 100% for RdRP; 65 and 77% for E; and 83 and 94% for the N RT-RPA assay. The assays were deployed to the field, where the RdRP RT-RPA assays confirmed to produce the most accurate results in three different laboratories in Africa (n = 89). The RPA assays were run in a mobile suitcase laboratory to facilitate the deployment at point of need. The assays can contribute to speed up the control measures as well as assist in the detection of COVID-19 cases in low-resource settings.
Conflict of interest statement
The authors declare the following competing financial interest(s): All authors except Marco Kaiser and Olfert Landt are in the public research sector. Mentioned authors are employed by GenExpress and/or Tib MolBiol, manufacturer of molecular standard or oligonucleotides. This does not alter the authors adherence to all the scientific policies on sharing data and materials.
Figures
Similar articles
-
Isothermal recombinase polymerase amplification-lateral flow detection of SARS-CoV-2, the etiological agent of COVID-19.J Virol Methods. 2021 Oct;296:114227. doi: 10.1016/j.jviromet.2021.114227. Epub 2021 Jul 2. J Virol Methods. 2021. PMID: 34224752 Free PMC article.
-
Development of a reverse transcription recombinase polymerase amplification assay for rapid and direct visual detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).PLoS One. 2021 Jan 6;16(1):e0245164. doi: 10.1371/journal.pone.0245164. eCollection 2021. PLoS One. 2021. PMID: 33406112 Free PMC article.
-
A multi-country phase 2 study to evaluate the suitcase lab for rapid detection of SARS-CoV-2 in seven Sub-Saharan African countries: Lessons from the field.J Clin Virol. 2023 May;162:105422. doi: 10.1016/j.jcv.2023.105422. Epub 2023 Mar 3. J Clin Virol. 2023. PMID: 36989731 Free PMC article. Clinical Trial.
-
Nucleic Acid Testing of SARS-CoV-2.Int J Mol Sci. 2021 Jun 7;22(11):6150. doi: 10.3390/ijms22116150. Int J Mol Sci. 2021. PMID: 34200331 Free PMC article. Review.
-
Application of recombinase polymerase amplification with lateral flow assay to pathogen point-of-care diagnosis.Front Cell Infect Microbiol. 2024 Nov 18;14:1475922. doi: 10.3389/fcimb.2024.1475922. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39624267 Free PMC article. Review.
Cited by
-
Harnessing recombinase polymerase amplification for rapid multi-gene detection of SARS-CoV-2 in resource-limited settings.Biosens Bioelectron. 2021 Oct 1;189:113328. doi: 10.1016/j.bios.2021.113328. Epub 2021 May 14. Biosens Bioelectron. 2021. PMID: 34051382 Free PMC article.
-
Development and Clinical Validation of RT-LAMP-Based Lateral-Flow Devices and Electrochemical Sensor for Detecting Multigene Targets in SARS-CoV-2.Int J Mol Sci. 2022 Oct 28;23(21):13105. doi: 10.3390/ijms232113105. Int J Mol Sci. 2022. PMID: 36361893 Free PMC article.
-
Toward a next-generation diagnostic tool: A review on emerging isothermal nucleic acid amplification techniques for the detection of SARS-CoV-2 and other infectious viruses.Anal Chim Acta. 2022 May 29;1209:339338. doi: 10.1016/j.aca.2021.339338. Epub 2021 Dec 1. Anal Chim Acta. 2022. PMID: 35569864 Free PMC article. Review.
-
Rapid Extraction and Detection of African Swine Fever Virus DNA Based on Isothermal Recombinase Polymerase Amplification Assay.Viruses. 2021 Aug 31;13(9):1731. doi: 10.3390/v13091731. Viruses. 2021. PMID: 34578312 Free PMC article.
-
Rapid Reverse Purification DNA Extraction Approaches to Identify Microbial Pathogens in Wastewater.Microorganisms. 2023 Mar 22;11(3):813. doi: 10.3390/microorganisms11030813. Microorganisms. 2023. PMID: 36985386 Free PMC article.
References
-
- WHO. WHO announces COVID-19 outbreak apandemic. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus... (accessed June 25, 2020).
-
- Corman V. M.; Landt O.; Kaiser M.; Molenkamp R.; Meijer A.; Chu D. K.; Bleicker T.; Brunink S.; Schneider J.; Schmidt M. L.; Mulders D. G.; Haagmans B. L.; van der Veer B.; van den Brink S.; Wijsman L.; Goderski G.; Romette J. L.; Ellis J.; Zambon M.; Peiris M.; Goossens H.; Reusken C.; Koopmans M. P.; Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 200004510.2807/1560-7917.ES.2020.25.3.2000045. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

