The Shaping of a B Cell Pool Maximally Responsive to Infections
- PMID: 33472004
- PMCID: PMC12203416
- DOI: 10.1146/annurev-immunol-042718-041238
The Shaping of a B Cell Pool Maximally Responsive to Infections
Abstract
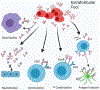
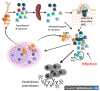
B cell subsets differ in development, tissue distribution, and mechanisms of activation. In response to infections, however, all can differentiate into extrafollicular plasmablasts that rapidly provide highly protective antibodies, indicating that these plasmablasts are the main humoral immune response effectors. Yet, the effectiveness of this response type depends on the presence of antigen-specific precursors in the circulating mature B cell pool, a pool that is generated initially through the stochastic processes of B cell receptor assembly. Importantly, germinal centers then mold the repertoire of this B cell pool to be increasingly responsive to pathogens by generating a broad array of antimicrobial memory B cells that act as highly effective precursors of extrafollicular plasmablasts. Such B cell repertoire molding occurs in two ways: continuously via the chronic germinal centers of mucosal lymphoid tissues, driven by the presence of the microbiome, and via de novo generated germinal centers following acute infections. For effectively evaluating humoral immunity as a correlate of immune protection, it might be critical to measure memory B cell pools in addition to antibody titers.
Keywords: B cell repertoire; B cell subsets; antibodies; extrafollicular foci; extrafollicular responses; germinal centers; host-pathogen interaction; immunity.
Figures

References
-
- Cedillo-Barrón L, García-Cordero J, Bustos-Arriaga J, León-Juárez M, Gutiérrez-Castañeda B. 2014. Antibody response to dengue virus. Microbes Infect 16: 711–20 - PubMed
-
- Williams KL, Sukupolvi-Petty S, Beltramello M, Johnson S, Sallusto F, Lanzavecchia A, Diamond MS, Harris E. 2013. Therapeutic efficacy of antibodies lacking Fcγ receptor binding against lethal dengue virus infection is due to neutralizing potency and blocking of enhancing antibodies [corrected]. PLoS Pathog 9: e1003157. - PMC - PubMed
-
- Baumgarth N 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 11: 34–46 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

