Deleterious variants in X-linked CFAP47 induce asthenoteratozoospermia and primary male infertility
- PMID: 33472045
- PMCID: PMC7895902
- DOI: 10.1016/j.ajhg.2021.01.002
Deleterious variants in X-linked CFAP47 induce asthenoteratozoospermia and primary male infertility
Abstract
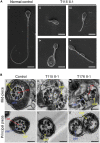
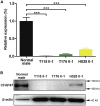
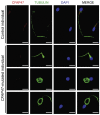
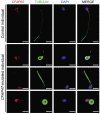
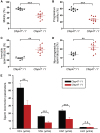
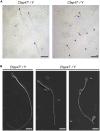
Asthenoteratozoospermia characterized by multiple morphological abnormalities of the flagella (MMAF) has been identified as a sub-type of male infertility. Recent progress has identified several MMAF-associated genes with an autosomal recessive inheritance in human affected individuals, but the etiology in approximately 40% of affected individuals remains unknown. Here, we conducted whole-exome sequencing (WES) and identified hemizygous missense variants in the X-linked CFAP47 in three unrelated Chinese individuals with MMAF. These three CFAP47 variants were absent in human control population genome databases and were predicted to be deleterious by multiple bioinformatic tools. CFAP47 encodes a cilia- and flagella-associated protein that is highly expressed in testis. Immunoblotting and immunofluorescence assays revealed obviously reduced levels of CFAP47 in spermatozoa from all three men harboring deleterious missense variants of CFAP47. Furthermore, WES data from an additional cohort of severe asthenoteratozoospermic men originating from Australia permitted the identification of a hemizygous Xp21.1 deletion removing the entire CFAP47 gene. All men harboring hemizygous CFAP47 variants displayed typical MMAF phenotypes. We also generated a Cfap47-mutated mouse model, the adult males of which were sterile and presented with reduced sperm motility and abnormal flagellar morphology and movement. However, fertility could be rescued by the use of intra-cytoplasmic sperm injections (ICSIs). Altogether, our experimental observations in humans and mice demonstrate that hemizygous mutations in CFAP47 can induce X-linked MMAF and asthenoteratozoospermia, for which good ICSI prognosis is suggested. These findings will provide important guidance for genetic counseling and assisted reproduction treatments.
Keywords: CFAP47; CFAP65; ICSI; MMAF; cilia; flagellum; male infertility; mice; sperm.
Copyright © 2021 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Moira K. O’Bryan is a member of the Monash IVF Research and Education Foundation steering committee. The other authors declare no competing interests.
Figures


References
-
- Matzuk M.M., Lamb D.J. Genetic dissection of mammalian fertility pathways. Nat. Cell Biol. 2002;4(Suppl):s41–s49. - PubMed
-
- Tournaye H., Krausz C., Oates R.D. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2017;5:544–553. - PubMed
-
- Shahrokhi S.Z., Salehi P., Alyasin A., Taghiyar S., Deemeh M.R. Asthenozoospermia: Cellular and molecular contributing factors and treatment strategies. Andrologia. 2020;52:e13463. - PubMed
-
- Ben Khelifa M., Coutton C., Zouari R., Karaouzène T., Rendu J., Bidart M., Yassine S., Pierre V., Delaroche J., Hennebicq S. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014;94:95–104. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

