Trophectoderm mechanics direct epiblast shape upon embryo implantation
- PMID: 33472064
- PMCID: PMC7816124
- DOI: 10.1016/j.celrep.2020.108655
Trophectoderm mechanics direct epiblast shape upon embryo implantation
Abstract
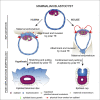
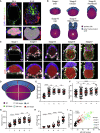
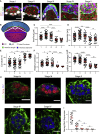
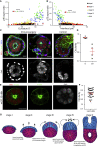
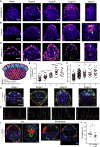
Implantation is a hallmark of mammalian embryogenesis during which embryos establish their contacts with the maternal endometrium, remodel, and undertake growth and differentiation. The mechanisms and sequence of events through which embryos change their shape during this transition are largely unexplored. Here, we show that the first extraembryonic lineage, the polar trophectoderm, is the key regulator for remodeling the embryonic epiblast. Loss of its function after immuno-surgery or inhibitor treatments prevents the epiblast shape transitions. In the mouse, the polar trophectoderm exerts physical force upon the epiblast, causing it to transform from an oval into a cup shape. In human embryos, the polar trophectoderm behaves in the opposite manner, exerting a stretching force. By mimicking this stretching behavior in mouse embryogenesis, we could direct the epiblast to adopt the disc-like shape characteristic of human embryos at this stage. Thus, the polar trophectoderm acts as a conserved regulator of epiblast shape.
Keywords: epiblast; morphogenesis; mouse/human implantation; tissue remodeling; trophectoderm.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Acampora D., Omodei D., Petrosino G., Garofalo A., Savarese M., Nigro V., Di Giovannantonio L.G., Mercadante V., Simeone A. Loss of the Otx2-Binding Site in the Nanog Promoter Affects the Integrity of Embryonic Stem Cell Subtypes and Specification of Inner Cell Mass-Derived Epiblast. Cell Rep. 2016;15:2651–2664. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

