Clinical course and challenging management of early COVID-19 infection after heart transplantation: case report of two patients
- PMID: 33472599
- PMCID: PMC7816134
- DOI: 10.1186/s12879-021-05793-6
Clinical course and challenging management of early COVID-19 infection after heart transplantation: case report of two patients
Abstract
Background: There are limited data on Coronavirus disease 2019 (COVID-19) in solid organ transplant patients, especially in heart transplant recipients, with only a few case reports and case series described so far. Heart transplant recipients may be at particular high risk due to their comorbidities and immunosuppressed state.
Case presentation: This report describes the clinical course and the challenging management of early COVID-19 infection in two heart transplant recipients who tested positive for the SARS-CoV-2 virus in the perioperative period of the transplant procedure. The two patients developed a severe form of the disease and ultimately died despite the initiation of an antiviral monotherapy with hydroxychloroquine coupled with the interruption of mycophenolate mofetil.
Conclusions: These two cases illustrate the severity and poor prognosis of COVID-19 in the perioperative period of a heart transplant. Thorough screening of donors and recipients is mandatory, and the issue of asymptomatic carriers needs to be addressed.
Keywords: Asymptomatic carrier; Case report; Coronavirus disease 2019 (COVID-19); Heart transplantation; Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


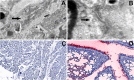
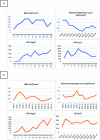
References
-
- Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;0(0) [cited 2020 Apr 25]. Available from: https://www.jhltonline.org/article/S1053-2498(20)31467-4/abstract. - PMC - PubMed
-
- Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. [cited 2020 Apr 27];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/ajt.15941. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

