Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial
- PMID: 33472855
- PMCID: PMC7815251
- DOI: 10.1136/bmj.n84
Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial
Abstract
Objective: To determine whether tocilizumab improves clinical outcomes for patients with severe or critical coronavirus disease 2019 (covid-19).
Design: Randomised, open label trial.
Setting: Nine hospitals in Brazil, 8 May to 17 July 2020.
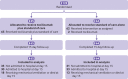
Participants: Adults with confirmed covid-19 who were receiving supplemental oxygen or mechanical ventilation and had abnormal levels of at least two serum biomarkers (C reactive protein, D dimer, lactate dehydrogenase, or ferritin). The data monitoring committee recommended stopping the trial early, after 129 patients had been enrolled, because of an increased number of deaths at 15 days in the tocilizumab group.
Interventions: Tocilizumab (single intravenous infusion of 8 mg/kg) plus standard care (n=65) versus standard care alone (n=64).
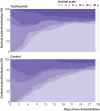
Main outcome measure: The primary outcome, clinical status measured at 15 days using a seven level ordinal scale, was analysed as a composite of death or mechanical ventilation because the assumption of odds proportionality was not met.
Results: A total of 129 patients were enrolled (mean age 57 (SD 14) years; 68% men) and all completed follow-up. All patients in the tocilizumab group and two in the standard care group received tocilizumab. 18 of 65 (28%) patients in the tocilizumab group and 13 of 64 (20%) in the standard care group were receiving mechanical ventilation or died at day 15 (odds ratio 1.54, 95% confidence interval 0.66 to 3.66; P=0.32). Death at 15 days occurred in 11 (17%) patients in the tocilizumab group compared with 2 (3%) in the standard care group (odds ratio 6.42, 95% confidence interval 1.59 to 43.2). Adverse events were reported in 29 of 67 (43%) patients who received tocilizumab and 21 of 62 (34%) who did not receive tocilizumab.
Conclusions: In patients with severe or critical covid-19, tocilizumab plus standard care was not superior to standard care alone in improving clinical outcomes at 15 days, and it might increase mortality.
Trial registration: ClinicalTrials.gov NCT04403685.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from hospitals and research institutes participating in the Coalition covid-19 Brazil, Fleury Laboratory in São Paulo, Brazil, and Instituto Votorantim for the submitted work. JAGGP reports support from Pfizer, Jansen, Sanofi, United Medical, MSD, Astellas, Astra Zeneca, and Eurofarma. DLCF has received grants from Abbvie, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Daichii Sankyo, GSK, Janssen, Takeda, Novartis, Pfizer, Sandoz, Sanofi, Viracta, Onconova, AGIOS, Astellas, MEDAC, Roche, Janssen, ABBVIE, Novartis, Takeda, Amgen, Libbs, Pfizer, Bristol Myers Squibb, Celgene, and Eurofarma. LKD receives a research grant from Bristol Myers Squibb, Roche, and Boehringer Ingelheim. RDL received grants and personal fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, and Bayer, and grants from Amgen, GlaxoSmithKline, Medtronic, and Sanofi Aventis outside the submitted work. OB received grants from AstraZeneca, Bayer, Pfizer, Novartis, Amgen, Boehringer Ingelheim, and Servier. DJBM reports support from Novartis. ANC reports receiving grants from Pfizer. AA reports receiving grants from Sanofi-Pasteur, Bayer, and Population Health Research Institute, and personal fees from Bayer, Boehringer Ingelheim, Novo Nordisk, and Lilly. ABC reports grants from Bactiguard, Ionis Pharmaceuticals, Brazilian Ministry of Health (PROADI-SUS), Brazilian Ministry of Science and Technology, Bayer, Pfizer, Hillrom, Fisher & Paykel, and Baxter. PS received grants from Roche, BioCryst, Amgen, Merck, Eurofarma, Novartis, Abbvie, Janssen, Alexion-Advisory, Novartis, Abbvie, Janssen, and Alexion.
Figures
Comment in
-
Covid-19 controversies: the tocilizumab chapter.BMJ. 2021 Jan 27;372:n244. doi: 10.1136/bmj.n244. BMJ. 2021. PMID: 33504502 No abstract available.
References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization, 2020. https://Covid19.who.int/. [Accessed 19 October 2020.]
-
- Jones L, Palumbo D, Brown D. Coronavirus: A visual guide to the economic impact. BBC News: BBC; 2020.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
