Cellular and Viral Determinants of HSV-1 Entry and Intracellular Transport towards Nucleus of Infected Cells
- PMID: 33472938
- PMCID: PMC8092704
- DOI: 10.1128/JVI.02434-20
Cellular and Viral Determinants of HSV-1 Entry and Intracellular Transport towards Nucleus of Infected Cells
Abstract
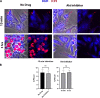
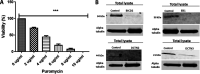
HSV-1 employs cellular motor proteins and modulates kinase pathways to facilitate intracellular virion capsid transport. Previously, we and others have shown that the Akt inhibitor miltefosine inhibited virus entry. Herein, we show that the protein kinase C inhibitors staurosporine (STS) and gouml inhibited HSV-1 entry into Vero cells, and that miltefosine prevents HSV-1 capsid transport toward the nucleus. We have reported that the HSV-1 UL37 tegument protein interacts with the dynein motor complex during virus entry and virion egress, while others have shown that the UL37/UL36 protein complex binds dynein and kinesin causing a saltatory movement of capsids in neuronal axons. Co-immoprecipitation experiments confirmed previous findings from our laboratory that the UL37 protein interacted with the dynein intermediate chain (DIC) at early times post infection. This UL37-DIC interaction was concurrent with DIC phosphorylation in infected, but not mock-infected cells. Miltefosine inhibited dynein phosphorylation when added before, but not after virus entry. Inhibition of motor accessory protein dynactins (DCTN2, DCTN3), the adaptor proteins EB1 and the Bicaudal D homolog 2 (BICD2) expression using lentiviruses expressing specific shRNAs, inhibited intracellular transport of virion capsids toward the nucleus of human neuroblastoma (SK-N-SH) cells. Co-immunoprecipitation experiments revealed that the major capsid protein Vp5 interacted with dynactins (DCTN1/p150 and DCTN4/p62) and the end-binding protein (EB1) at early times post infection. These results show that Akt and kinase C are involved in virus entry and intracellular transport of virion capsids, but not in dynein activation via phosphorylation. Importantly, both the UL37 and Vp5 viral proteins are involved in dynein-dependent transport of virion capsids to the nuclei of infected cells.Importance. Herpes simplex virus type-1 enter either via fusion at the plasma membranes or endocytosis depositing the virion capsids into the cytoplasm of infected cells. The viral capsids utilize the dynein motor complex to move toward the nuclei of infected cells using the microtubular network. This work shows that inhibitors of the Akt kinase and kinase C inhibit not only viral entry into cells but also virion capsid transport toward the nucleus. In addition, the work reveals that the virion protein ICP5 (VP5) interacts with the dynein cofactor dynactin, while the UL37 protein interacts with the dynein intermediate chain (DIC). Importantly, neither Akt nor Kinase C was found to be responsible for phosphorylation/activation of dynein indicating that other cellular or viral kinases may be involved.
Copyright © 2021 American Society for Microbiology.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

