Comparison of Subgenomic and Total RNA in SARS-CoV-2 Challenged Rhesus Macaques
- PMID: 33472939
- PMCID: PMC8103707
- DOI: 10.1128/JVI.02370-20
Comparison of Subgenomic and Total RNA in SARS-CoV-2 Challenged Rhesus Macaques
Abstract
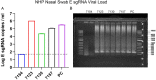
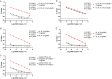
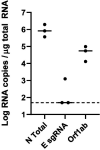
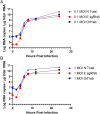
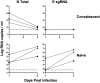
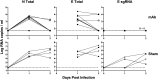
Respiratory virus challenge studies involve administration of the challenge virus and sampling to assess for protection from the same anatomical locations. It can therefore be difficult to differentiate actively replicating virus from input challenge virus. For SARS-CoV-2, specific monitoring of actively replicating virus is critical to investigate the protective and therapeutic efficacy of vaccines, monoclonal antibodies, and antiviral drugs. We developed a SARS-CoV-2 subgenomic RNA (sgRNA) RT-PCR assay to differentiate productive infection from inactivated or neutralized virus. Subgenomic RNAs are generated after cell entry and are poorly incorporate into mature virions, and thus may provide a marker for actively replicating virus. We show envelope (E) sgRNA was degraded by RNase in infected cell lysates, while genomic RNA (gRNA) was protected, presumably due to packaging into virions. To investigate the capacity of the sgRNA assay to distinguish input challenge virus from actively replicating virus in vivo, we compared the E sgRNA assay to a standard nucleoprotein (N) or E total RNA assay in convalescent rhesus macaques and in antibody-treated rhesus macaques after experimental SARS-CoV-2 challenge. In both studies, the E sgRNA assay was negative, suggesting protective efficacy, whereas the N and E total RNA assays remained positive. These data suggest the potential utility of sgRNA to monitor actively replicating virus in prophylactic and therapeutic SARS-CoV-2 studies.ImportanceDeveloping therapeutic and prophylactic countermeasures for the SARS-CoV-2 virus is a public health priority. During challenge studies, respiratory viruses are delivered and sampled from the same anatomical location. It is therefore important to distinguish actively replicating virus from input challenge virus. The most common assay for detecting SARS-CoV-2 virus, reverse transcription polymerase chain reaction (RT-PCR) targeting nucleocapsid total RNA, cannot distinguish neutralized input virus from replicating virus. In this study, we assess SARS-CoV-2 subgenomic RNA as a potential measure of replicating virus in rhesus macaques.
Copyright © 2021 Dagotto et al.
Figures

References
-
- Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Nakano S, Sun L, Fulcher ML, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O'Neal WK, Randell SH, Boucher RC, Baric RS. 2020. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182:429–446. doi: 10.1016/j.cell.2020.05.042. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

