Dissecting human embryonic skeletal stem cell ontogeny by single-cell transcriptomic and functional analyses
- PMID: 33473154
- PMCID: PMC8249634
- DOI: 10.1038/s41422-021-00467-z
Dissecting human embryonic skeletal stem cell ontogeny by single-cell transcriptomic and functional analyses
Abstract
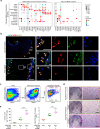
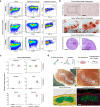
Human skeletal stem cells (SSCs) have been discovered in fetal and adult long bones. However, the spatiotemporal ontogeny of human embryonic SSCs during early skeletogenesis remains elusive. Here we map the transcriptional landscape of human limb buds and embryonic long bones at single-cell resolution to address this fundamental question. We found remarkable heterogeneity within human limb bud mesenchyme and epithelium, and aligned them along the proximal-distal and anterior-posterior axes using known marker genes. Osteo-chondrogenic progenitors first appeared in the core limb bud mesenchyme, which give rise to multiple populations of stem/progenitor cells in embryonic long bones undergoing endochondral ossification. Importantly, a perichondrial embryonic skeletal stem/progenitor cell (eSSPC) subset was identified, which could self-renew and generate the osteochondral lineage cells, but not adipocytes or hematopoietic stroma. eSSPCs are marked by the adhesion molecule CADM1 and highly enriched with FOXP1/2 transcriptional network. Interestingly, neural crest-derived cells with similar phenotypic markers and transcriptional networks were also found in the sagittal suture of human embryonic calvaria. Taken together, this study revealed the cellular heterogeneity and lineage hierarchy during human embryonic skeletogenesis, and identified distinct skeletal stem/progenitor cells that orchestrate endochondral and intramembranous ossification.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

