Natural history, response to systemic therapy, and genomic landscape of plasmacytoid urothelial carcinoma
- PMID: 33473164
- PMCID: PMC8007750
- DOI: 10.1038/s41416-020-01244-2
Natural history, response to systemic therapy, and genomic landscape of plasmacytoid urothelial carcinoma
Erratum in
-
Correction: Natural history, response to systemic therapy, and genomic landscape of plasmacytoid urothelial carcinoma.Br J Cancer. 2022 May;126(8):1236. doi: 10.1038/s41416-022-01721-w. Br J Cancer. 2022. PMID: 35145255 Free PMC article. No abstract available.
Abstract
Background: Plasmacytoid urothelial carcinoma (PUC) is a rare, aggressive histologic variant of urothelial cancer characterised by a diffuse growth pattern and CDH1 mutation. We studied the efficacy of preoperative platinum-based chemotherapy in nonmetastatic PUC and immune checkpoint inhibitors (ICIs) in advanced PUC.
Methods: Cases of nonmetastatic PUC and advanced PUC treated with ICIs at our institution were identified. Outcomes were compared to those of a published cohort of patients with urothelial carcinoma not otherwise specified.
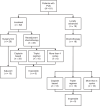
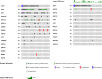
Results: We identified 81 patients with nonmetastatic PUC. Of the patients with localised disease who underwent neoadjuvant chemotherapy, pathologic complete response and downstaging rates were 12 and 21%, respectively. Pathologic downstaging was not associated with significant improvement in clinical outcomes. Up to 18% of localised disease and 28% of locally advanced cases had unresectable disease at the time of surgery. ICI-treated advanced PUC (N = 21) had progression-free and overall survival of 4.5 and 10.5 months, respectively, and a 38% response rate. FGFR3 and DNA damage response gene alterations were observed in 3 and 15% of cases, respectively.
Conclusions: PUC is associated with high disease burden and poor chemosensitivity. Increased awareness and recognition of this disease variant will allow for new treatment strategies.
Conflict of interest statement
MYT has received Research Support from Bristol Myers Squibb, Clovis and Pharmacyclics; DBS has consulted with/received honoraria from Pfizer, Loxo Oncology, Lilly Oncology, BridgeBio, Vividion Therapeutics, Scorpion Therapeutics and Illumina; SAF has received research support from AstraZeneca, Genentech/Roche, is a consultant/advisory board member for Merck, and owns stock in Urogen, Allogene Therapeutics, Neogene Therapeutics, Kronos Bio, and Inconovir; DFB has received research funding from Novartis, has received personal fees from Merck Sharp & Dohme, Eisai, Fidia Farmaceutici S.p.A., Lilly, and UroGen Pharma; and has received grants and personal fees from Bristol‐Myers Squibb, Roche/Genentech, and Novartis; and grants from Dendreon; GVI has received personal fees from Mirati Therapeutics and Janssen and research support from Novartis; JER has consulted for AstraZeneca, Bayer, Merck, BMS, Roche, Genentech, Seattle Genetics, Astellas, Boehringher Ingelheim, GSK, Mirati, Janssen, Lilly, and Pfizer. He has also received funding for clinical trials from Roche/Genentech, AstraZeneca, Bayer, Seattle Genetics and Astellas.
Figures

Comment in
-
Urological Oncology: Bladder, Penis and Urethral Cancer, and Basic Principles of Oncology.J Urol. 2022 Sep;208(3):734-736. doi: 10.1097/JU.0000000000002807. Epub 2022 Jun 21. J Urol. 2022. PMID: 35727637 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

