A pathogenic UFSP2 variant in an autosomal recessive form of pediatric neurodevelopmental anomalies and epilepsy
- PMID: 33473208
- PMCID: PMC8105169
- DOI: 10.1038/s41436-020-01071-z
A pathogenic UFSP2 variant in an autosomal recessive form of pediatric neurodevelopmental anomalies and epilepsy
Abstract
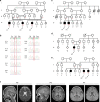
Purpose: Neurodevelopmental disabilities are common and genetically heterogeneous. We identified a homozygous variant in the gene encoding UFM1-specific peptidase 2 (UFSP2), which participates in the UFMylation pathway of protein modification. UFSP2 variants are implicated in autosomal dominant skeletal dysplasias, but not neurodevelopmental disorders. Homozygosity for the variant occurred in eight children from four South Asian families with neurodevelopmental delay and epilepsy. We describe the clinical consequences of this variant and its effect on UFMylation.
Methods: Exome sequencing was used to detect potentially pathogenic variants and identify shared regions of homozygosity. Immunoblotting assessed protein expression and post-translational modifications in patient-derived fibroblasts.
Results: The variant (c.344T>A; p.V115E) is rare and alters a conserved residue in UFSP2. Immunoblotting in patient-derived fibroblasts revealed reduced UFSP2 abundance and increased abundance of UFMylated targets, indicating the variant may impair de-UFMylation rather than UFMylation. Reconstituting patient-derived fibroblasts with wild-type UFSP2 reduced UFMylation marks. Analysis of UFSP2's structure indicated that variants observed in skeletal disorders localize to the catalytic domain, whereas V115 resides in an N-terminal domain possibly involved in substrate binding.
Conclusion: Different UFSP2 variants cause markedly different diseases, with homozygosity for V115E causing a severe syndrome of neurodevelopmental disability and epilepsy.
Conflict of interest statement
R.J.D. is a member of the Scientific Advisory Board for Agios Pharmaceuticals and Vida Ventures.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

