Striatal activity topographically reflects cortical activity
- PMID: 33473213
- PMCID: PMC7612253
- DOI: 10.1038/s41586-020-03166-8
Striatal activity topographically reflects cortical activity
Abstract
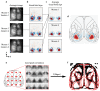
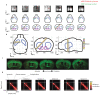
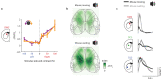
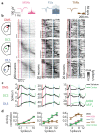
The cortex projects to the dorsal striatum topographically1,2 to regulate behaviour3-5, but spiking activity in the two structures has previously been reported to have markedly different relations to sensorimotor events6-9. Here we show that the relationship between activity in the cortex and striatum is spatiotemporally precise, topographic, causal and invariant to behaviour. We simultaneously recorded activity across large regions of the cortex and across the width of the dorsal striatum in mice that performed a visually guided task. Striatal activity followed a mediolateral gradient in which behavioural correlates progressed from visual cue to response movement to reward licking. The summed activity in each part of the striatum closely and specifically mirrored activity in topographically associated cortical regions, regardless of task engagement. This relationship held for medium spiny neurons and fast-spiking interneurons, whereas the activity of tonically active neurons differed from cortical activity with stereotypical responses to sensory or reward events. Inactivation of the visual cortex abolished striatal responses to visual stimuli, supporting a causal role of cortical inputs in driving the striatum. Striatal visual responses were larger in trained mice than untrained mice, with no corresponding change in overall activity in the visual cortex. Striatal activity therefore reflects a consistent, causal and scalable topographical mapping of cortical activity.
Conflict of interest statement
The authors declare no competing interests.
Figures











Comment in
-
Reflected responses.Nat Rev Neurosci. 2021 Apr;22(4):194-195. doi: 10.1038/s41583-021-00439-7. Nat Rev Neurosci. 2021. PMID: 33558743 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

