Survey of spiking in the mouse visual system reveals functional hierarchy
- PMID: 33473216
- PMCID: PMC10399640
- DOI: 10.1038/s41586-020-03171-x
Survey of spiking in the mouse visual system reveals functional hierarchy
Abstract
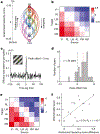
The anatomy of the mammalian visual system, from the retina to the neocortex, is organized hierarchically1. However, direct observation of cellular-level functional interactions across this hierarchy is lacking due to the challenge of simultaneously recording activity across numerous regions. Here we describe a large, open dataset-part of the Allen Brain Observatory2-that surveys spiking from tens of thousands of units in six cortical and two thalamic regions in the brains of mice responding to a battery of visual stimuli. Using cross-correlation analysis, we reveal that the organization of inter-area functional connectivity during visual stimulation mirrors the anatomical hierarchy from the Allen Mouse Brain Connectivity Atlas3. We find that four classical hierarchical measures-response latency, receptive-field size, phase-locking to drifting gratings and response decay timescale-are all correlated with the hierarchy. Moreover, recordings obtained during a visual task reveal that the correlation between neural activity and behavioural choice also increases along the hierarchy. Our study provides a foundation for understanding coding and signal propagation across hierarchically organized cortical and thalamic visual areas.
Figures











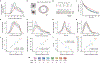

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

