Advances in the development of personalized neoantigen-based therapeutic cancer vaccines
- PMID: 33473220
- PMCID: PMC7816749
- DOI: 10.1038/s41571-020-00460-2
Advances in the development of personalized neoantigen-based therapeutic cancer vaccines
Abstract
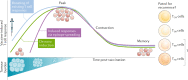
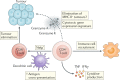
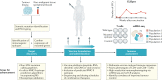
Within the past decade, the field of immunotherapy has revolutionized the treatment of many cancers with the development and regulatory approval of various immune-checkpoint inhibitors and chimeric antigen receptor T cell therapies in diverse indications. Another promising approach to cancer immunotherapy involves the use of personalized vaccines designed to trigger de novo T cell responses against neoantigens, which are highly specific to tumours of individual patients, in order to amplify and broaden the endogenous repertoire of tumour-specific T cells. Results from initial clinical studies of personalized neoantigen-based vaccines, enabled by the availability of rapid and cost-effective sequencing and bioinformatics technologies, have demonstrated robust tumour-specific immunogenicity and preliminary evidence of antitumour activity in patients with melanoma and other cancers. Herein, we provide an overview of the complex process that is necessary to generate a personalized neoantigen vaccine, review the types of vaccine-induced T cells that are found within tumours and outline strategies to enhance the T cell responses. In addition, we discuss the current status of clinical studies testing personalized neoantigen vaccines in patients with cancer and considerations for future clinical investigation of this novel, individualized approach to immunotherapy.
Conflict of interest statement
P.A.O. has received research funding from and has been an adviser of Amgen, Armo BioSciences, Array, AstraZeneca/MedImmune, Bristol-Myers Squibb, Celldex, CytomX, Merck, Neon Therapeutics, Novartis, Pfizer and Roche/Genentech. E.B. declares no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

