Complex natural product production methods and options
- PMID: 33474503
- PMCID: PMC7803631
- DOI: 10.1016/j.synbio.2020.12.001
Complex natural product production methods and options
Abstract
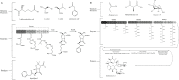
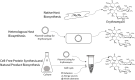
Natural products have had a major impact upon quality of life, with antibiotics as a classic example of having a transformative impact upon human health. In this contribution, we will highlight both historic and emerging methods of natural product bio-manufacturing. Traditional methods of natural product production relied upon native cellular host systems. In this context, pragmatic and effective methodologies were established to enable widespread access to natural products. In reviewing such strategies, we will also highlight the development of heterologous natural product biosynthesis, which relies instead on a surrogate host system theoretically capable of advanced production potential. In comparing native and heterologous systems, we will comment on the base organisms used for natural product biosynthesis and how the properties of such cellular hosts dictate scaled engineering practices to facilitate compound distribution. In concluding the article, we will examine novel efforts in production practices that entirely eliminate the constraints of cellular production hosts. That is, cell free production efforts will be introduced and reviewed for the purpose of complex natural product biosynthesis. Included in this final analysis will be research efforts made on our part to test the cell free biosynthesis of the complex polyketide antibiotic natural product erythromycin.
Keywords: Bio-manufacturing; Biosynthesis; Heterologous host; Native host; Natural product.
© 2021 Production and hosting by Elsevier B.V. on behalf of KeAi Communications Co.
Conflict of interest statement
The Authors Declare No Conflicts.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

