Total IgE as a Marker for Chronic Spontaneous Urticaria
- PMID: 33474856
- PMCID: PMC7840871
- DOI: 10.4168/aair.2021.13.2.206
Total IgE as a Marker for Chronic Spontaneous Urticaria
Abstract
Objective: Immunoglobulin E (IgE) and its receptor, FcɛRI, importantly contribute to the pathophysiology of chronic spontaneous urticaria (CSU). Recent findings point to a possible role of total IgE as a marker of CSU disease activity, endotypes, and responses to treatment. The evidence in support of total IgE included in the diagnostic workup of patients with CSU has not yet been reviewed.
Methods: Publications were searched via PubMed. The search terms used were "chronic urticaria" and "total IgE." Studies were screened by titles and abstracts, and 141 were used in the review.
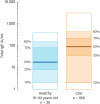
Results: CSU patients frequently had elevated total IgE serum levels (up to 50%), but normal or very low total IgE levels also occurred. High total IgE may represent high disease activity, longer disease duration, high chance of responding to omalizumab treatment, quick relapse after stopping omalizumab, and lower chance of responding to cyclosporine. Low IgE, in contrast, may suggest Type IIb autoimmune CSU, poor response to treatment with omalizumab and a better chance to benefits from cyclosporine treatment. Furthermore, IgE in different CSU cohorts may have different physicochemical properties that could explain differences in treatment responses to IgE-directed therapies.
Conclusion: The results of our review suggest that total IgE is a valuable marker for CSU, and we recommend its assessment in the routine diagnostic workup of CSU patients.
Keywords: Urticaria; biomarkers; chronic spontaneous urticaria; cyclosporine; diagnosis; immunoglobulin E; omalizumab; receptor; therapeutics.
Copyright © 2021 The Korean Academy of Asthma, Allergy and Clinical Immunology · The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
Sabine Altrichter is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, AstraZeneca, Moxie, Sanofi and ThermoFisher.
Figures

References
-
- Fricke J, Ávila G, Keller T, Weller K, Lau S, Maurer M, et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. 2020;75:423–432. - PubMed
-
- Saini S, Shams M, Bernstein JA, Maurer M. Urticaria and angioedema across the ages. J Allergy Clin Immunol Pract. 2020;8:1866–1874. - PubMed
-
- Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. - PubMed
-
- Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunol Rev. 2018;282:232–247. - PubMed
-
- Maurer M, Giménez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med. 2019;381:1321–1332. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

