Clinical Characteristics, Urinary Leukotriene E4 Levels, and Aspirin Desensitization Results in Patients With NSAID-Induced Blended Reactions
- PMID: 33474858
- PMCID: PMC7840864
- DOI: 10.4168/aair.2021.13.2.229
Clinical Characteristics, Urinary Leukotriene E4 Levels, and Aspirin Desensitization Results in Patients With NSAID-Induced Blended Reactions
Abstract
Purpose: Data on non-steroidal anti-inflammatory drug (NSAID) hypersensitivity in Southeast Asia are scarce. Increased urinary leukotriene E4 (uLTE4) levels have been suggested as a biomarker of NSAID-exacerbated respiratory disease (NERD). This study investigated clinical patterns of NSAID sensitivity in Thailand and the diagnostic roles of uLTE4 measurement in various phenotypes.
Methods: The clinical phenotypes in 92 Thai adults with cross-reactive NSAID hypersensitivity were characterized based on the clinical history and drug provocation. The uLTE4 levels were measured at baseline, after aspirin provocation and after desensitization.
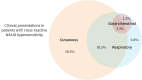
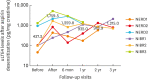
Results: More than half of the patients (56.5%) presented with cutaneous symptoms (NSAID-exacerbated cutaneous disease), while one-third (33.7%) developed symptoms in at least 2 systems (NSAID-induced blended reactions; NIBR). Fifty-two patients underwent drug provocation and 59.6% of them yielded positive results. After drug provocation, a significant number of patients with confirmed NSAID cross-reactivity experienced clinical symptoms in more than one organ system. The uLTE4 levels at baseline were comparable between the NSAID-tolerant and NSAID-sensitive groups, but were substantially increased after aspirin provocation predominantly in NERD (983.4 pg/mg creatinine) and NIBR (501.0 pg/mg creatinine) compared to NSAID-tolerant subjects (122.1 pg/mg creatinine, P < 0.01 and 0.05, respectively). The uLTE4 levels were elevated after aspirin desensitization, although nasal polyposis and asthma were under control in 3 NERD and 3 NIBR subjects.
Conclusions: NIBR is not uncommon among NSAID-sensitive patients in Thailand. The diagnostic value of basal uLTE4 levels was limited, but increased uLTE4 levels upon aspirin provocation suggest NSAID cross-reactivity with respiratory components. This study indicates that aspirin desensitization, if necessary, might be effective in both NERD and NIBR.
Trial registration: ClinicalTrials.gov Identifier: NCT03849625.
Keywords: Leukotriene E4; anti-inflammatory agents; aspirin; biomarkers; desensitization; drug hypersensitivity; non-steroidal; phenotype.
Copyright © 2021 The Korean Academy of Asthma, Allergy and Clinical Immunology · The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures


References
-
- Cahill KN, Laidlaw TM. Pathogenesis of aspirin-induced reactions in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2016;36:681–691. - PubMed
-
- Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum. 2002;46:2201–2206. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

