Long-term Therapeutic Success of Intravenous Rituximab in 26 Patients with Indolent Primary Cutaneous B-cell Lymphoma
- PMID: 33475146
- PMCID: PMC9366677
- DOI: 10.2340/00015555-3746
Long-term Therapeutic Success of Intravenous Rituximab in 26 Patients with Indolent Primary Cutaneous B-cell Lymphoma
Abstract
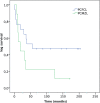
Systemic monotherapy with rituximab is a well-known treatment approach for primary cutaneous follicle centre lymphoma and primary cutaneous marginal zone lymphoma. Both have excellent prognosis despite high relapse rates. To investigate the long-term effectiveness and clinical outcome of intravenous rituximab at a dose of 375 mg/m2 once weekly, data for 26 patients (17 primary cutaneous follicle centre lymphoma and 9 primary cutaneous marginal zone lymphoma) were analysed retrospectively. Complete remissions occurred in 20 (77%) and partial remissions in 6 patients (23%), demonstrating an overall response rate of 100%. The relapse rate was 52.9% in primary cutaneous follicle centre lymphoma and 88.9% in primary cutaneous marginal zone lymphoma. Ongoing complete remissions after therapy with rituximab were observed in 9 patients (34.6%) with a median progression-free survival of 161 months (13.4 years). These results confirm that intravenous rituximab is an effective and well-tolerated treatment with durable responses in a relevant percentage of patients at a median follow-up of 148 months (12.3 years).
Keywords: follow-up; intravenous rituximab therapy; primary cutaneous follicle centre lymphoma; primary cutaneous marginal zone lymphoma; primary cutaneous B-cell lymphoma.
Conflict of interest statement
Figures
Similar articles
-
Rituximab monotherapy for primary cutaneous B-cell lymphoma: response and follow-up in 16 patients.Ann Oncol. 2009 Feb;20(2):326-30. doi: 10.1093/annonc/mdn636. Epub 2008 Oct 3. Ann Oncol. 2009. PMID: 18836086
-
Intralesional and intravenous treatment of cutaneous B-cell lymphomas with the monoclonal anti-CD20 antibody rituximab: report and follow-up of eight cases.Br J Dermatol. 2006 Dec;155(6):1197-200. doi: 10.1111/j.1365-2133.2006.07523.x. Br J Dermatol. 2006. PMID: 17107389
-
Long-term outcome of intravenous therapy with rituximab in patients with primary cutaneous B-cell lymphomas.Br J Dermatol. 2013 Nov;169(5):1126-32. doi: 10.1111/bjd.12484. Br J Dermatol. 2013. PMID: 23796422
-
Rituximab in the treatment of primary cutaneous B-cell lymphoma: a review.Actas Dermosifiliogr. 2014 Jun;105(5):438-45. doi: 10.1016/j.ad.2012.10.021. Epub 2013 Mar 26. Actas Dermosifiliogr. 2014. PMID: 23540593 Review. English, Spanish.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
Cited by
-
How to treat primary cutaneous B cell lymphoma - Results from a monocentric cohort study on 98 patients.J Dtsch Dermatol Ges. 2025 Jul;23(7):822-830. doi: 10.1111/ddg.15702. Epub 2025 May 30. J Dtsch Dermatol Ges. 2025. PMID: 40445595 Free PMC article.
-
Treatment of Indolent Cutaneous B-Cell Lymphoma with Intralesional or Intravenous Rituximab.Cancers (Basel). 2022 Sep 30;14(19):4787. doi: 10.3390/cancers14194787. Cancers (Basel). 2022. PMID: 36230709 Free PMC article.
-
An adolescent with primary cutaneous follicle center lymphoma: a case report and literature review.Front Oncol. 2023 Nov 1;13:1273719. doi: 10.3389/fonc.2023.1273719. eCollection 2023. Front Oncol. 2023. PMID: 38023243 Free PMC article.
-
MHC-I upregulation safeguards neoplastic T cells in the skin against NK cell-mediated eradication in mycosis fungoides.Nat Commun. 2024 Jan 25;15(1):752. doi: 10.1038/s41467-024-45083-8. Nat Commun. 2024. PMID: 38272918 Free PMC article.
References
-
- Zinzani PL, Quaglino P, Pimpinelli N, Berti E, Baliva G, Rupoli S, et al. . Prognostic factors in primary cutaneous B-cell lymphoma: the Italian Study Group for Cutaneous Lymphomas. J Clin Oncol 2006; 24: 1376–1382. - PubMed
-
- Bogle MA, Riddle CC, Triana EM, Jones D, Duvic M. Primary cutaneous B-cell lymphoma. J Am Acad Dermatol 2005; 53: 479–484. - PubMed
-
- Wilcox RA. Cutaneous B-cell lymphomas: 2019 update on diagnosis, risk stratification, and management. Am J Hematol 2018; 93: 1427–1430. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases