Compartmentation of β2 -adrenoceptor stimulated cAMP responses by phosphodiesterase types 2 and 3 in cardiac ventricular myocytes
- PMID: 33475150
- PMCID: PMC8493936
- DOI: 10.1111/bph.15382
Compartmentation of β2 -adrenoceptor stimulated cAMP responses by phosphodiesterase types 2 and 3 in cardiac ventricular myocytes
Abstract
Background and purpose: In cardiac myocytes, cyclic AMP (cAMP) produced by both β1 - and β2 -adrenoceptors increases L-type Ca2+ channel activity and myocyte contraction. However, only cAMP produced by β1 -adrenoceptors enhances myocyte relaxation through phospholamban-dependent regulation of the sarco/endoplasmic reticulum Ca2+ ATPase 2 (SERCA2). Here we have tested the hypothesis that stimulation of β2 -adrenoceptors produces a cAMP signal that is unable to reach SERCA2 and determine what role, if any, phosphodiesterase (PDE) activity plays in this compartmentation.
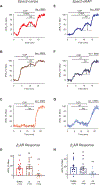
Experimental approach: The cAMP responses produced by β1 -and β2 -adrenoceptor stimulation were studied in adult rat ventricular myocytes using two different fluorescence resonance energy transfer (FRET)-based biosensors, the Epac2-camps, which is expressed uniformly throughout the cytoplasm of the entire cell and the Epac2-αKAP, which is targeted to the SERCA2 signalling complex.
Key results: Selective activation of β1 - or β2 -adrenoceptors produced cAMP responses detected by Epac2-camps. However, only stimulation of β1 -adrenoceptors produced a cAMP response detected by Epac2-αKAP. Yet, stimulation of β2 -adrenoceptors was able to produce a cAMP signal detected by Epac2-αKAP in the presence of selective inhibitors of PDE2 or PDE3, but not PDE4.
Conclusion and implications: These results support the conclusion that cAMP produced by β2 -adrenoceptor stimulation was not able to reach subcellular locations where the SERCA2 pump is located. Furthermore, this compartmentalized response is due at least in part to PDE2 and PDE3 activity. This discovery could lead to novel PDE-based therapeutic treatments aimed at correcting cardiac relaxation defects associated with certain forms of heart failure.
Keywords: cAMP; compartmentation; phosphodiesterase; ventricular myocyte; β-adrenergic receptors.
© 2021 The British Pharmacological Society.
Conflict of interest statement
Conflict of Interest Statement
None
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
Figures




Similar articles
-
Caveolae compartmentalise β2-adrenoceptor signals by curtailing cAMP production and maintaining phosphatase activity in the sarcoplasmic reticulum of the adult ventricular myocyte.J Mol Cell Cardiol. 2012 Feb;52(2):388-400. doi: 10.1016/j.yjmcc.2011.06.014. Epub 2011 Jun 26. J Mol Cell Cardiol. 2012. PMID: 21740911 Free PMC article.
-
Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling.Circ Res. 2006 Nov 10;99(10):1084-91. doi: 10.1161/01.RES.0000250046.69918.d5. Epub 2006 Oct 12. Circ Res. 2006. PMID: 17038640
-
Heart failure leads to altered β2-adrenoceptor/cyclic adenosine monophosphate dynamics in the sarcolemmal phospholemman/Na,K ATPase microdomain.Cardiovasc Res. 2019 Mar 1;115(3):546-555. doi: 10.1093/cvr/cvy221. Cardiovasc Res. 2019. PMID: 30165515 Free PMC article.
-
Myocardial phosphodiesterases and regulation of cardiac contractility in health and cardiac disease.Cardiovasc Drugs Ther. 2007 Jun;21(3):171-94. doi: 10.1007/s10557-007-6014-6. Epub 2007 Mar 21. Cardiovasc Drugs Ther. 2007. PMID: 17373584 Review.
-
The role of cardiac beta1- and beta2-adrenoceptor stimulation in heart failure.J Cardiovasc Pharmacol. 1990;16 Suppl 5:S133-7. J Cardiovasc Pharmacol. 1990. PMID: 11527117 Review.
Cited by
-
Compartmentalized cAMP signaling in cardiac ventricular myocytes.Cell Signal. 2022 Jan;89:110172. doi: 10.1016/j.cellsig.2021.110172. Epub 2021 Oct 20. Cell Signal. 2022. PMID: 34687901 Free PMC article. Review.
-
Cardiac Hypertrophy Changes Compartmentation of cAMP in Non-Raft Membrane Microdomains.Cells. 2021 Mar 3;10(3):535. doi: 10.3390/cells10030535. Cells. 2021. PMID: 33802377 Free PMC article.
-
Mitochondrial A-kinase anchoring proteins in cardiac ventricular myocytes.Physiol Rep. 2021 Sep;9(17):e15015. doi: 10.14814/phy2.15015. Physiol Rep. 2021. PMID: 34514737 Free PMC article.
-
AKAP12 Upregulation Associates With PDE8A to Accelerate Cardiac Dysfunction.Circ Res. 2024 Apr 12;134(8):1006-1022. doi: 10.1161/CIRCRESAHA.123.323655. Epub 2024 Mar 20. Circ Res. 2024. PMID: 38506047 Free PMC article.
-
Microtubule-Mediated Regulation of β2AR Translation and Function in Failing Hearts.Circ Res. 2023 Nov 10;133(11):944-958. doi: 10.1161/CIRCRESAHA.123.323174. Epub 2023 Oct 23. Circ Res. 2023. PMID: 37869877 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

