A dynamic model of the body gas stores for carbon dioxide, oxygen, and inert gases that incorporates circulatory transport delays to and from the lung
- PMID: 33475459
- PMCID: PMC8354828
- DOI: 10.1152/japplphysiol.00764.2020
A dynamic model of the body gas stores for carbon dioxide, oxygen, and inert gases that incorporates circulatory transport delays to and from the lung
Abstract
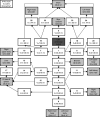
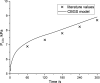
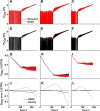
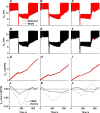
Many models of the body's gas stores have been generated for specific purposes. Here, we seek to produce a more general purpose model that: 1) is relevant for both respiratory (CO2 and O2) and inert gases; 2) is based firmly on anatomy and not arbitrary compartments; 3) can be scaled to individuals; and 4) incorporates arterial and venous circulatory delays as well as tissue volumes so that it can reflect rapid transients with greater precision. First, a "standard man" of 11 compartments was produced, based on data compiled by the International Radiation Protection Commission. Each compartment was supplied via its own parallel circulation, the arterial and venous volumes of which were based on reported tissue blood volumes together with data from a detailed anatomical model for the large arteries and veins. A previously published model was used for the blood gas chemistry of CO2 and O2. It was not permissible ethically to insert pulmonary artery catheters into healthy volunteers for model validation. Therefore, validation was undertaken by comparing model predictions with previously published data and by comparing model predictions with experimental data for transients in gas exchange at the mouth following changes in alveolar gas composition. Overall, model transients were fastest for O2, intermediate for CO2, and slowest for N2. There was good agreement between model estimates and experimentally measured data. Potential applications of the model include estimation of closed-loop gain for the ventilatory chemoreflexes and improving the precision associated with multibreath washout testing and respiratory measurement of cardiac output.NEW & NOTEWORTHY A model for the body gas stores has been generated that is applicable to both respiratory gases (CO2 and O2) and inert gases. It is based on anatomical details for organ volumes and blood contents together with anatomical details of the large arteries. It can be scaled to the body size and composition of different individuals. The model enables mixed venous gas compositions to be predicted from the systemic arterial compositions.
Keywords: mixed venous composition; mixed venous saturation; pulmonary arterial blood gases.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

