RET mutated C-cells proliferate more rapidly than non-mutated neoplastic cells
- PMID: 33475524
- PMCID: PMC7983519
- DOI: 10.1530/EC-20-0589
RET mutated C-cells proliferate more rapidly than non-mutated neoplastic cells
Abstract
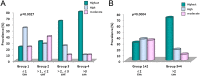
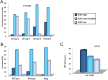
A statistically significant higher prevalence of the RET p.Met918Thr somatic mutation, identified by direct sequencing, was previously reported in MTC > 2 cm than in smaller tumors. Aim of this study was to correlate the full RET and RAS mutation profile, identified by a Next Generation Sequencing approach, with the growth rate, proliferation and tumor size of MTC. Data of 149 sporadic MTC patients were correlated with RET mutations and Ki67 positivity. Eighty-one cases had a somatic RET mutation, 40 had a RAS mutation and 28 were negative. A statistically significant higher prevalence of RET mutations was found in MTC > 2 cm. A higher prevalence of RET more aggressive mutations, higher allelic frequencies and, higher percentage of Ki67 positive cells were found in larger tumors which had also a worse outcome. Our study highlights the predominant role of RET somatic mutations in MTC tumorigenesis. We demonstrate that RET mutation prevalence and allelic frequency (AF) are significantly higher in larger tumors. Based on these results, we can conclude that RET mutated C-cells's growth and proliferation are more rapid than those of non-mutated cells and give origin to bigger and more aggressive MTC.
Keywords: Ki67; RAS; RET; allelic frequency; cells’ growth; medullary thyroid cancer.
Figures


Similar articles
-
The prevalence of somatic RAS mutations in medullary thyroid cancer - a Polish population study.Endokrynol Pol. 2015;66(2):121-5. doi: 10.5603/EP.2015.0018. Endokrynol Pol. 2015. PMID: 25931041
-
New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations.J Med Genet. 2016 Nov;53(11):729-734. doi: 10.1136/jmedgenet-2016-103833. Epub 2016 Jul 28. J Med Genet. 2016. PMID: 27468888
-
High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas.J Clin Endocrinol Metab. 2011 May;96(5):E863-8. doi: 10.1210/jc.2010-1921. Epub 2011 Feb 16. J Clin Endocrinol Metab. 2011. PMID: 21325462
-
RAS proto-oncogene in medullary thyroid carcinoma.Endocr Relat Cancer. 2015 Oct;22(5):R235-52. doi: 10.1530/ERC-15-0070. Endocr Relat Cancer. 2015. PMID: 26285815 Review.
-
Diversity of mutations in the RET proto-oncogene and its oncogenic mechanism in medullary thyroid cancer.Crit Rev Clin Lab Sci. 2016 Aug;53(4):217-27. doi: 10.3109/10408363.2015.1129529. Epub 2016 Jan 27. Crit Rev Clin Lab Sci. 2016. PMID: 26678667 Review.
Cited by
-
Limited Thyroidectomy Achieves Equivalent Survival to Total Thyroidectomy for Early Localized Medullary Thyroid Cancer.Cancers (Basel). 2024 Dec 4;16(23):4062. doi: 10.3390/cancers16234062. Cancers (Basel). 2024. PMID: 39682246 Free PMC article.
-
Allele frequency in thyroid cancer: mechanisms, challenges, and applications in cancer therapy.Thyroid Res. 2025 May 6;18(1):19. doi: 10.1186/s13044-025-00237-8. Thyroid Res. 2025. PMID: 40325461 Free PMC article. Review.
-
Higher RET Gene Expression Levels Do Not Represent anAlternative RET Activation Mechanism in Medullary Thyroid Carcinoma.Biomolecules. 2021 Oct 19;11(10):1542. doi: 10.3390/biom11101542. Biomolecules. 2021. PMID: 34680178 Free PMC article.
References
-
- Agrawal N, Jiao Y, Sausen M, Leary R, Bettegowda C, Roberts NJ, Bhan S, Ho AS, Khan Z, Bishop Jet al. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. Journal of Clinical Endocrinology and Metabolism 2013. 98 E364–E36. (10.1210/jc.2012-2703) - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

