Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial
- PMID: 33475736
- PMCID: PMC7819761
- DOI: 10.1182/blood.2020007535
Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial
Abstract
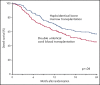
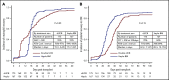
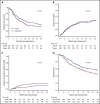
Results of 2 parallel phase 2 trials of transplantation of unrelated umbilical cord blood (UCB) or bone marrow (BM) from HLA-haploidentical relatives provided equipoise for direct comparison of these donor sources. Between June 2012 and June 2018, 368 patients aged 18 to 70 years with chemotherapy-sensitive lymphoma or acute leukemia in remission were randomly assigned to undergo UCB (n = 186) or haploidentical (n = 182) transplant. Reduced-intensity conditioning comprised total-body irradiation with cyclophosphamide and fludarabine for both donor types. Graft-versus-host disease prophylaxis for UCB transplantation was cyclosporine and mycophenolate mofetil (MMF) and for haploidentical transplantation, posttransplant cyclophosphamide, tacrolimus, and MMF. The primary end point was 2-year progression-free survival (PFS). Treatment groups had similar age, sex, self-reported ethnic origin, performance status, disease, and disease status at randomization. Two-year PFS was 35% (95% confidence interval [CI], 28% to 42%) compared with 41% (95% CI, 34% to 48%) after UCB and haploidentical transplants, respectively (P = .41). Prespecified analysis of secondary end points recorded higher 2-year nonrelapse mortality after UCB, 18% (95% CI, 13% to 24%), compared with haploidentical transplantation, 11% (95% CI, 6% to 16%), P = .04. This led to lower 2-year overall survival (OS) after UCB compared with haploidentical transplantation, 46% (95% CI, 38-53) and 57% (95% CI 49% to 64%), respectively (P = .04). The trial did not demonstrate a statistically significant difference in the primary end point, 2-year PFS, between the donor sources. Although both donor sources extend access to reduced-intensity transplantation, analyses of secondary end points, including OS, favor haploidentical BM donors. This trial was registered at www.clinicaltrials.gov as #NCT01597778.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Comment in
-
Haploidentical transplantation: finally, some light.Blood. 2021 Jan 21;137(3):296-297. doi: 10.1182/blood.2020008767. Blood. 2021. PMID: 33475743 No abstract available.
References
-
- Szydlo R, Goldman JM, Klein JP, et al. . Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767-1777. - PubMed
-
- Lee SJ, Klein J, Haagenson M, et al. . High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576-4583. - PubMed
-
- Barker JN, Weisdorf DJ, DeFor TE, et al. . Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343-1347. - PubMed
-
- Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13(2):132-132. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

