Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association With Survival
- PMID: 33475752
- PMCID: PMC7821027
- DOI: 10.1001/jamanetworkopen.2020.21869
Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association With Survival
Abstract
Importance: There exists considerable biological and clinical variability between histologic variants of metastatic renal cell carcinoma (mRCC). Data reporting on patterns of metastasis in histologic variants of mRCC are sparse.
Objective: To characterize sites of metastasis and their association with survival across the 3 most common histologic variants of mRCC: clear cell (ccRCC), papillary (pRCC), and chromophobe (chrRCC).
Design, setting, and participants: In this multicenter, international cohort study, the International mRCC Database Consortium (IMDC) database was used to identify consecutive patients starting systemic therapy for mRCC between 2002 and 2019. Patients with mixed histologic subtype were excluded. Statistical analysis was performed from February to June 2020.
Exposures: Data regarding histologic subtype and sites of metastatic involvement at the time of first systemic therapy initiation were collected.
Main outcomes and measures: The primary outcomes were prevalence of metastatic site involvement and overall survival (OS) from time of systemic therapy initiation. Patients with multiple sites of metastatic involvement were included in analyses of all groups to which they had metastases.
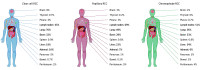
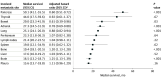
Results: A total of 10 105 patients were eligible for analysis. Median (interquartile range) age at diagnosis was 60 (53-67) years, 7310 (72.4%) were men and 8526 (84.5%) underwent nephrectomy. Of these, 9252 (92%) had ccRCC, 667 (7%) had pRCC, and 186 (2%) had chrRCC. The median number of sites of metastasis was 2 (range, 0-7). In ccRCC, the most common sites of metastasis were lung (70%; 6189 of 8804 patients [448 missing]), lymph nodes (45%; 3874 of 8655 patients [597 missing]), bone (32%; 2847 of 8817 patients [435 missing]), liver (18%; 1560 of 8804 [448 missing]), and adrenal gland (10%; 678 of 6673 patients [2579 missing]). Sites of metastasis varied between subtypes. Lung, adrenal, brain, and pancreatic metastases were more frequent in ccRCC, lymph node involvement was more common in pRCC, and liver metastases were more frequent in chrRCC. Median OS for ccRCC varied by site of metastatic involvement, ranging between 16 months (95% CI, 13.7-18.8 months) for the pleura and 50 months (95% CI, 41.1-55.5 months) for the pancreas. Compared with ccRCC, patients with pRCC tended to have lower OS, regardless of metastatic site.
Conclusions and relevance: Sites of metastatic involvement differ according to histologic subtype in mRCC and are associated with OS. These data highlight the clinical and biological variability between histologic subtypes of mRCC. Patterns of metastatic spread may reflect differences in underlying disease biology. Further work to investigate differences in immune, molecular, and genetic profiles between metastatic sites and histologic subtypes is encouraged.
Conflict of interest statement
Figures
Comment in
-
Urological Oncology: Adrenal, Renal, Ureteral and Retroperitoneal Tumors.J Urol. 2021 Aug;206(2):472-474. doi: 10.1097/JU.0000000000001857. Epub 2021 May 13. J Urol. 2021. PMID: 33983046 No abstract available.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

