The effect of synthetic bone graft substitutes on bone formation in rabbit calvarial defects
- PMID: 33475862
- PMCID: PMC7819904
- DOI: 10.1007/s10856-020-06483-6
The effect of synthetic bone graft substitutes on bone formation in rabbit calvarial defects
Abstract
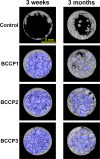
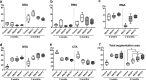
The aim of this study was to evaluate the influence of the intensity of the biomimetic hydroxyapatite (HA) coating of α-tricalcium phosphate (α-TCP) on biomaterial degradation and bone formation. Twenty-four female NZW rabbits of approximately 12 weeks of age were used. Critical size defects were randomly treated with 3%:97% HA:α-TCP (BBCP1), 12%:88% HA:α-TCP (BBCP2), and 23%:77% HA:α-TCP (BBCP3), respectively or sham. All defects were covered with a resorbable collagen membrane. Animals were euthanized after 3 and 12 weeks of healing and samples were investigated by micro-CT and histologic analysis. Ingrowth of newly formed woven bone from the original bone at 3-week healing period was observed in all samples. At the 12-week healing period, the new bone in the peripheral area was mainly lamellar and in the central region composed of both woven and lamellar bone. New bony tissue was found on the surface of all three types of granules and at the interior of the BBCP1 granules. Samples with 3% HA showed significantly less residual biomaterial in comparison to the other two groups. Furthermore, BBCP1 significantly promoted new bone area as compared to other three groups and more bone volume as compared to the control. Within its limitations, this study indicated the highest degradation rate in case of BBCP1 concomitant with the highest rate of bone formation. Hence, formation of new bone can be affected by the level of biomimetic HA coating of α-TCP.
Conflict of interest statement
The authors have no financial interest to declare in relation to the content of this article. Mr Claudio Zihlmann is employed by Geistlich Pharma AG (Wolhusen, Switzerland).
Figures









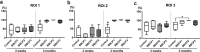


References
-
- Moy P, Palacci P. Minor bone augmentation procedures. In: Palacci P, Ericsson I, editors. Esthetic implant dentistry. Soft and hard tissue management. Quintessence Publishing Co: IL.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

