Efficacy, safety, and tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain: a randomized, double-blind, placebo-controlled study
- PMID: 33475984
- PMCID: PMC8149545
- DOI: 10.1007/s40199-020-00381-6
Efficacy, safety, and tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain: a randomized, double-blind, placebo-controlled study
Abstract
Background: Breakthrough pain (BTP) is an important challenge in treatment and requires a rapid onset of action for pain control. BTP should be adequately controlled with a stable dose of a short-acting oral opioid. So far, no drug is available for the treatment of BTP in cancer patients in Iran, so we designed the first study in Iran to investigate the effect of sublingual fentanyl in relief of pain episodes in these patients.
Objective: The purpose of this study was to evaluate the efficacy and safety of sublingual fentanyl in the treatment of breakthrough pain in cancer patients.
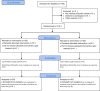
Method: This study was a randomized double-blind placebo-controlled clinical trial in cancer patients with breakthrough pain (at least 1-4 episodes of acute pain with moderate to severe pain daily) referred to the pain clinic of Akhtar and Masih Daneshvari hospitals in 2019. The study consisted of two stages: 100 patients were selected by simple, non-random sampling and entered the open-label titration phase. The primary efficacy endpoint was the sum of pain intensity difference over 30 min post-administration. Secondary efficacy endpoints included pain intensity difference (PID) and pain relief (PR) throughout the 60-min post-dose assessment period. In the double-blind study, patients were randomly divided into two groups of placebo (n=50) and intervention (sublingual fentanyl tablet) (n=50). For evaluation of efficacy, 10 episodes were treated in each group and the results were recorded by the patient. (Clinical trial registration: IRCT20131124015515N8).
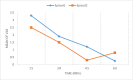
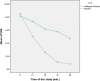
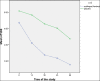
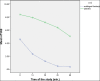
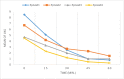
Results: A total of 100 patients entered the titration phase, primary efficacy of sublingual fentanyl was 3.5±0.6 and secondary efficacy of sublingual fentanyl (60 min, after treatment) was 0.3±0.6 which was statistically significant. In the titration phase, the treatment success rate was 100%. In the double-blind phase of the study, the pain intensity in multiple episodes showed a significant improvement at 15, 30, 45, and 60 min after drug administration (P=0.0001). The intensity of pain in each episode was significantly decreased compared to the next episode (P=0.0001). The mean frequency of pain episodes in the sublingual fentanyl group showed a significant decrease (P=0.0001). The most common adverse drug events in the titration phase were drowsiness (20%), dizziness (7%), and nausea 4%, and in the double-blind phase only drowsiness (12%). (Cancer Research Center, Shahid Beheshti University of Medical Sciences, Survey).
Conclusion: Sublingual fentanyl appears to be effective for patients with rapid-onset analgesia, has short-acting duration, is effective medication, safe, and well tolerated. It is a suitable choice in Iranian patients with chronic cancer-related pain controlled suffering from acute pain episodes related to cancer.
Keywords: Breakthrough pain; Cancer; Sublingual fentanyl; Treatment.
Conflict of interest statement
The authors claim no conflict of interest.
Figures
Similar articles
-
Fentanyl buccal tablet for the relief of breakthrough pain in opioid-tolerant adult patients with chronic neuropathic pain: a multicenter, randomized, double-blind, placebo-controlled study.Clin Ther. 2007 Apr;29(4):588-601. doi: 10.1016/j.clinthera.2007.04.007. Clin Ther. 2007. PMID: 17617282 Clinical Trial.
-
Efficacy and long-term tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain.Curr Med Res Opin. 2009 Dec;25(12):2877-85. doi: 10.1185/03007990903368310. Curr Med Res Opin. 2009. PMID: 19814586 Clinical Trial.
-
Efficacy and safety of sublingual fentanyl orally disintegrating tablet at doses determined by titration for the treatment of breakthrough pain in Japanese cancer patients: a multicenter, randomized, placebo-controlled, double-blind phase III trial.Int J Clin Oncol. 2015 Feb;20(1):198-206. doi: 10.1007/s10147-014-0697-z. Epub 2014 May 20. Int J Clin Oncol. 2015. PMID: 24839047 Clinical Trial.
-
Fentanyl buccal tablet.Drugs Today (Barc). 2008 Jan;44(1):41-54. doi: 10.1358/dot.2008.44.1.1178469. Drugs Today (Barc). 2008. PMID: 18301803 Review.
-
Fentanyl sublingual: in breakthrough pain in opioid-tolerant adults with cancer.Drugs. 2010 Dec 3;70(17):2281-8. doi: 10.2165/11200910-000000000-00000. Drugs. 2010. PMID: 21080744 Review.
Cited by
-
Improving the Emergency Department Management of Sickle Cell Vaso-Occlusive Pain Crisis: The Role and Options of Sublingual and Intranasally Administered Analgesia.J Clin Med Res. 2023 Jan;15(1):10-22. doi: 10.14740/jocmr4841. Epub 2023 Jan 24. J Clin Med Res. 2023. PMID: 36755761 Free PMC article. Review.
-
Effectiveness of fentanyl buccal soluble film in cancer patients with inadequate breakthrough pain control.BMC Palliat Care. 2024 Jun 14;23(1):150. doi: 10.1186/s12904-024-01483-7. BMC Palliat Care. 2024. PMID: 38877477 Free PMC article.
References
-
- Hanks G, Cherny NI, Fallon M: Opioid analgesic therapy. In Oxford Textbook of Palliative Medicine, edn 3. Edited by Doyle D, Hanks G, Cherny N, Calman K. Oxford: Oxford University Press; 2004: 316–341.
-
- Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. The management of cancer-related breakthrough pain: recommendations of a task group of the science Committee of Association for palliative medicine of Great Britain and Ireland. Eur J Pain. 2009;13:331–338. doi: 10.1016/j.ejpain.2008.06.014. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

