Pharmacokinetics of meropenem in children with sepsis undergoing extracorporeal life support: A prospective observational study
- PMID: 33476064
- PMCID: PMC8248190
- DOI: 10.1111/jcpt.13344
Pharmacokinetics of meropenem in children with sepsis undergoing extracorporeal life support: A prospective observational study
Abstract
What is known and objective: Meropenem, a broad-spectrum carbapenem, is frequently used to treat severe bacterial infections in critically ill children. Recommendations for meropenem doses in adult infections are available; however, few studies have been published regarding the use of meropenem in children with sepsis, especially in those receiving continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO). We aimed to investigate the pharmacokinetic (PK) parameters of meropenem in children with sepsis receiving extracorporeal life support (ECLS).
Methods: This was a prospective observational clinical study of children with sepsis receiving ECMO or CRRT in the paediatric intensive care unit (PICU) of a children's hospital. The enrolled children received 20 mg/kg meropenem infusion over 1 hour, every 8 hours, and were grouped into children receiving ECMO, children receiving CRRT and children receiving neither ECMO nor CRRT. Plasma meropenem concentrations were determined using a validated high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). The key PK parameters were determined using the non-compartmental approach.
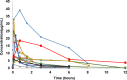
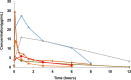
Results and discussion: Twenty-seven patients were finally enrolled. The eCLCR of the CRRT group was lower than that of the ECMO group. The values of elimination half-life (t1/2 ), area under the plasma concentration-time curve (AUCtau ), area under the plasma concentration-time curve from time zero to infinity (AUC0-∞ ), and total clearance (CL) in the ECMO group were not different from those of the other groups (all p > 0.05). However, the AUCtau (p = 0.0137) and AUC0-∞ (p = 0.0234) significantly decreased after filtration through a hemofiltration membrane in patients receiving CRRT.
What is new and conclusion: No significant alterations in the PK parameters of meropenem occurred in children with sepsis administered ECMO and/or CRRT. Further investigations including PK modelling could provide evidence for appropriate meropenem dosing regimens during ECLS administration.
Keywords: AN69 membrane; children; continuous renal replacement therapy; critically ill; extracorporeal life support; extracorporeal membrane oxygenation; meropenem; pharmacokinetic; sepsis.
© 2021 The Authors. Journal of Clinical Pharmacy and Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Figures

References
-
- Levy Hara G, Kanj SS, Pagani L, et al. Ten key points for the appropriate use of antibiotics in hospitalised patients: a consensus from the Antimicrobial Stewardship and Resistance Working Groups of the International Society of Chemotherapy. Int J Antimicrob Agents. 2016;48:239‐246. - PubMed
-
- Kumar A. Early antimicrobial therapy in severe sepsis and septic shock. Curr Infect Dis Rep. 2010;12:336‐344. - PubMed
-
- Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529‐535. - PubMed
-
- MacArthur RD, Miller M, Albertson T, et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis. 2004;38:284‐288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

